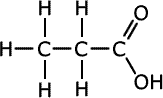
Higher-level only
This section looks at polymeric substances that are made using condensation reactions. These types of polymer differ from addition polymers in that there are nearly always two types of monomer that must be combined.
Students should be familiar with organic structures and reactions of carboxylic acids and alcohols before studying this section.
Syllabus ref: S2.4.6Structure 2.4.6 - Condensation polymers form by the reaction between functional groups in each monomer with the release of a small molecule. (HL)
- Represent the repeating unit of polyamides and polyesters from given monomer structures.
Guidance
- All biological macromolecules form by condensation reactions and break down by hydrolysis.
Tools and links
- Structure 3.2 - What functional groups in molecules can enable them to act as monomers for condensation reactions?
Condensation reactions
Condensation reactions are chemical processes where two molecules combine, resulting in the elimination of a smaller molecule, commonly water, but also includes hydrogen chloride or ammonia. This type of reaction is fundamental in the formation of various types of polymers and plays a crucial role in both synthetic chemistry and biological systems.
In synthetic chemistry, condensation reactions are key to producing polymers such as polyesters and nylons. These polymers are created when monomers, containing functional groups like carboxylic acids, alcohols, or amines, react together, releasing water or other small molecules as by-products. The ability to control the reaction conditions allows chemists to synthesize materials with specific properties and applications.
Condensation reactions
In biological systems, condensation reactions are essential for forming long-chain biomolecules. For example, the formation of proteins from amino acids or nucleic acids from nucleotides involves condensation reactions, where water is released as the monomers bond to form polymers. These reactions are fundamental to various life processes, including the synthesis of enzymes, structural proteins, and genetic material.
The significance of condensation reactions extends beyond polymer production. They are also crucial in the synthesis of small molecules in organic chemistry, including esters and amides, which are vital in pharmaceuticals, agrochemicals, and other industrial applications. Understanding and manipulating these reactions is key to advancing material science, medicine, and biotechnology.
Condensation polymers
These were first developed in the mid-20th Century by joining monomer units together using condensation (addition-elimination) reactions.
A classic example of a condensation polymer is nylon, made from the reaction between a diamine and a dicarboxylic acid. Similarly, polyesters, another common type of condensation polymer, are synthesized from dicarboxylic acids and diols. These polymers are notable for their strength, durability, and various applications, ranging from textiles to engineering plastics.
Condensation polymers are also significant in the natural world. Proteins, formed by the condensation of amino acids, and polysaccharides, such as cellulose and starch, developed from sugar monomers, are fundamental to biological structures and processes. These natural condensation polymers play crucial roles in the structure and function of living organisms.
The process of creating condensation polymers allows for the manipulation of molecular structure to achieve desired properties, making them highly versatile in both industrial and biomedical applications. However, environmental concerns over their degradation and recyclability have spurred research into more sustainable alternatives and improved recycling methods.
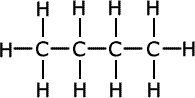
Nylon and polyamides
Nylon and polyamides are synthetic polymers widely recognized for their strength, elasticity, and resistance to abrasion and chemicals. Their synthesis involves condensation polymerization, typically of a diamine with a dicarboxylic acid, resulting in the release of water molecules. This process creates long chains of repeating amide units, characteristic of polyamides. An alternate method uses a single monomer with both amine and acid groups, producing the same polymer chain through internal condensation.
Condensation polymers - Nylon
Nylon, the first commercially successful synthetic thermoplastic polymer, was developed in the 1930s. It revolutionized the textile industry with its introduction as a silk substitute, particularly in products like stockings and parachutes. Beyond textiles, nylon's properties make it ideal for a wide range of applications, including automotive parts, electrical equipment, and consumer goods. Its durability, resistance to heat and chemicals, and low moisture absorbency contribute to its versatility.
Polyamides, encompassing various types of nylons and other similar polymers, are used extensively in engineering and manufacturing. Their applications range from high-performance fibers in the automotive and aerospace industries to components in electronics and machinery. The ability to blend polyamides with other materials or modify their structure allows for the creation of composites with enhanced properties, meeting specific requirements of diverse applications.
The synthesis and use of nylon and polyamides illustrate the significant impact of synthetic polymers in modern technology and everyday life. Their ongoing development and adaptation continue to offer innovative solutions across various sectors.
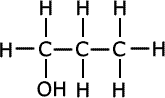
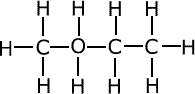
Terylene and polyesters
Terylene, a well-known polyester, is synthesized through a condensation reaction involving ethylene glycol and terephthalic acid. This process, known as esterification, results in the formation of ester bonds, linking the monomers together into long polymer chains. During the synthesis, water is released as a byproduct, hence the term condensation polymerization. Terylene, also commonly referred to as Dacron or PET (polyethylene terephthalate), is a thermoplastic polymer, meaning it can be melted and reshaped multiple times without significantly altering its properties.
Condensation polymers - Polyesters
Polyesters, including Terylene, have a wide array of applications due to their desirable properties such as durability, resistance to chemicals and wrinkles, and ease of care. In the textile industry, Terylene is used for making clothing, home furnishings like curtains and bed sheets, and industrial textiles such as conveyor belts and fire hoses. Its strength and resistance to stretching and shrinking make it an ideal fabric for various uses.
Beyond textiles, polyesters are used in packaging, particularly in the form of PET bottles, which are lightweight, strong, and recyclable. They also find applications in making high-strength ropes, sails for yachts, tapes, and films. In the automotive and aerospace industries, polyester resins reinforced with fibers like glass are used to make composites for body panels and other structural components. The versatility and adaptability of polyesters like Terylene continue to make them valuable in numerous industrial and consumer applications.
Amino acids and polypeptides
Amino acids, the building blocks of proteins, are organic compounds characterized by two functional groups: an amino group (NH2) and a carboxyl group (COOH). Each amino acid also contains a side chain, denoted as R, which varies among different amino acids and determines their unique properties. The central carbon atom (alpha carbon) is bonded to the amino group, carboxyl group, a hydrogen atom, and the R group.
The linking of amino acids to form polypeptides occurs through a condensation reaction, specifically a peptide bond formation. This process involves the amino group of one amino acid reacting with the carboxyl group of another, resulting in the release of a water molecule. The resulting covalent bond, known as a peptide bond, connects the carbon atom of the carboxyl group of one amino acid to the nitrogen atom of the amino group of the next.
As more amino acids join this growing chain, a polypeptide is formed. The sequence of amino acids in the polypeptide chain, governed by the genetic code, determines the structure and function of the resulting protein. The side chains of the amino acids influence the polypeptide's folding and shape, crucial for its biological activity. Proteins can consist of one or more polypeptide chains, intricately folded and twisted into specific three-dimensional structures essential for their function in living organisms.
Hydrolysis of condensation polymers
Hydrolysis of condensation polymers is a chemical process that breaks down polymer chains into their monomeric units using water. This reaction essentially reverses the condensation polymerization process. In hydrolysis, a water molecule is used to cleave the chemical bonds (like ester or amide bonds) in the polymer, resulting in the separation of the monomers.
This process is significant because it enables the depolymerization of polymers like polyesters, nylons, and polyamides, which were originally formed by the elimination of water or other small molecules. During hydrolysis, the water molecule provides a hydroxyl group (–OH) and a hydrogen atom (H), which attach to the ends of the broken bond in the polymer chain. As a result, the polymer chain is shortened, gradually breaking down into its constituent monomers or smaller oligomers.
Hydrolysis of condensation polymers can be catalyzed by acids, bases, or enzymes, depending on the type of polymer and the desired speed of the reaction. This process is not only important in industrial recycling and waste management of synthetic polymers but also plays a crucial role in biological systems. For example, in the human body, enzymes catalyze the hydrolysis of proteins (polypeptides) into amino acids, which are then utilized in various physiological processes.
Worked examples
Q271-01 Select the substance with the higher boiling point in each of the following pairs. Explain your reasoning:- C2H6 and C3H8
- CH3CH2OH and CH3OCH3
|
In the first pair of compounds there are no dipoles so the intermolecular forces are due to dispersion attractions only. The larger of the two molecules has the greater intermolecular force. Therefore C3H8 has the higher boiling point. In the second pair of compounds their relative masses are the same, but the alcohol, CH3CH2OH, has hydrogen bonding due to the OH group, whereas the ether (methoxymethane) has only weak dipole - dipole interactions. The alcohol, CH3CH2OH, has the higher boiling point. |
Q271-02 Which substance has the highest boiling point?
- CH4
- He
- HF
- Cl2
|
Q271-03 The very high heat of vaporization of H2O is mainly a result of
- dispersion forces
- covalent bonds
- inter-ionic attractions
- hydrogen bonding
|
The enthalpy of vaporisation is the amount of energy needed to turn one mole of a substance from liquid into vapour. It is a measure of the strength of the intermolecular forces. Water's high enthalpy of vaporisation is due to the strengthof intermolecular forces caused by hydrogen bonding. |
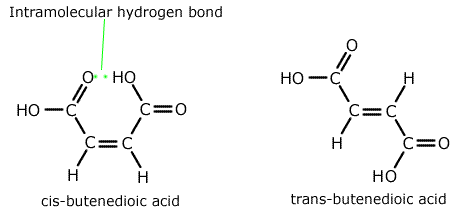
Q271-04 Explain the following statement: "Cis-butenedioic acid has a lower melting point than its isomer trans-butenedioic acid."
Answer
|
The lower melting point of the cis-butenedioic acid is indicative of the weaker intermolecular forces compared to the trans isomer. To understand why it is necessary to look at the structure of each isomer.
|
Q271-05 Arrange the following compounds in order of increasing boiling point and explain your choice by reference to the intermolecular forces involved: Bromoethene, chloroethene, ethene
Answer
|
Ethene, CH2=CH2, is a non-polar simple molecular substance with a low boiling point. However, both bromoethene, CH2=CHBr and chloroethene, CH2=CHCl are polar substances with similar polarity. Bromoethene has a much larger relative mass than chloroethene and so larger dispersion forces. This is the deciding factor, leaving the order of increasing boiling point as ethene < chloroethene < bromoethene. |
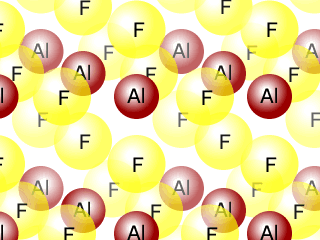
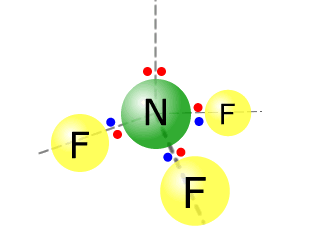
Q271-06 Aluminium fluoride AlF3 is a solid up to temperatures of 1250ºC, whereas nitrogen trifluoride is a gas above -129ºC. Describe the bonding and structure in samples of each of these substances.
Answer
|
Aluminium fluoride has a giant ionic structure, whereas nitrogen trifluoride has a simple molecular structure.
|
Q271-07 Which statements are generally true about the melting points of substances?
- I - Melting points are higher for compounds containing ions than for compounds containing molecules.
- II - A compound with a low melting point is less volatile than a compound with a higher melting point.
- III - The melting point of a compound is decreases by the presence of impurities.
- I only
- I and III only
- II and III only
- I, II and III
|
I - Melting points are higher for compounds containing ions than
for compounds containing molecules - true Correct response I and III only |
Q271-08 CH3COCH3 is the first member of the ketone homologous series. Draw the full structural formula of the next member of the homologous series and predict how its melting point compares with that of CH3COCH3
Answer
|
Members of homologous series differ by one -CH2- unit. The first member of the ketones (alkanones) is propanone, thus the second member is butanone, CH3COCH2CH3.
|
Q271-09 The compounds A, B and C have approximately the same molar mass:
| A | B | C |
| C4H10 | CH3CH2CH2OH | CH3OCH2CH3 |
When the compounds are arranged in order of increasing boiling points (lowest
boiling point first), the correct order is:
- A, C, B
- A, B, C
- B, C, A
- C, B, A
|
The three compounds belong to different homologous series, alkanes, alcohols and ethers. As they have the same relative mass we can discount the effect of dispersion forces. Alkanes are non-polar and have very low melting and boiling points. Ethers are weakly polar and have higher boiling points than alkanes. Alcohols have extensive hydrogen bonding and the highest boiling point of the three groups. The correct order of increasing boiling point is A, C, B.
|
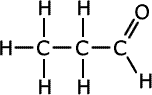
Q271-10 Identify the strongest type of intermolecular force present in each of the compounds propan-1-ol, propanal and propanoic acid. List these compounds in decreasing order of boiling point.
Answer
|
The three compounds belong to different homologous series, alcohols, aldehydes (alkanals) and carboxylic acids (alkanoic acids). Although all three compounds have different relative masses, they are not extremely different and the effect of the functional groups is greater than that of dispersion forces. In propanoic acid there is extensive hydrogen bonding and so it has the highest boiling point. The next highest boiling point is in propan-1-ol which also has hydrogen bonding. The aldehyde has only weaker dipole-dipole interactions.
|