Higher-level only
The first row of the 'd' block elements excluding scandium and zinc are known as transition metals. They share common characteristics that arise from having similar atomic and ionic radii.
Syllabus ref: S3.1.10Structure 3.1.10 - Transition element complexes are coloured due to the absorption of light when an electron is promoted between the orbitals in the split d-sublevels. The colour absorbed is complementary to the colour observed. (HL)
- Apply the colour wheel to deduce the wavelengths and frequencies of light absorbed and/or observed.
Guidance
- Students are not expected to know the different splitting patterns and their relation to the coordination number.
- The colour wheel and the equation c = λ f are given in the data booklet.
Tools and links
- Reactivity 3.4 - What is the nature of the reaction between transition element ions and ligands in forming complex ions?
- Tool 1, Inquiry 2 - How can colorimetry or spectrophotometry be used to calculate the concentration of a solution of coloured ions?
The nature of colour
Colour in materials or compounds is caused when the light reaching the eyes has some of the wavelengths removed by absorption. When we see an object as red it is only this colour that is reflected from the object while the other colours or wavelengths are absorbed.
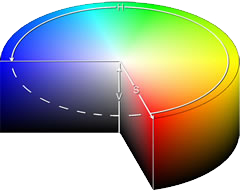
A coloured solution is caused by the white light passing through it and losing some of its wavelengths by absorption. If the solution appears blue it means that the complementary colours are absorbed by the solution.
In the complementary colour cylinder at the left, the colour that is opposite is the colour that is seen when one is absorbed.
For example, absorption of red light would leave the turquoise (cyan) colour showing. (image: wikipedia)
Coloured compounds
If colour is caused by the absorption of certain wavelengths from white light, the question remains - how are these wavelengths absorbed?
The colour in the transition metals (d-block) is usually due to the 'splitting' of the 'd' shell orbitals into slightly different energy levels. As a result, certain wavelengths of energy can be absorbed by the d-block elements (with electrons jumping between these slightly different energy levels), resulting in the complementary colour being observed.
The theory that explains the production of colour in this way is called crystal, or ligand field, theory.
This is not the only mechanism by which colour can be produced in compounds; there is also charge transfer and conjugation. Charge transfer involves the transfer of electrons from ligands to the transition metal orbitals and often produces intense colours.
One example is the deep purple colour of the manganate(VII) ion.
Conjugation is alternate double and single bonds in (usually) organic molecules, producing delocalised orbitals whose electrons can absorb light at visible wavelengths.
The azo-dyes are a good example, as are chromophores such as chlorophyll.