Standard level
The radius of a circle or sphere is the distance from the centre to the outer edge.
Syllabus ref: S3.1.3Structure 3.1.3 - Periodicity refers to trends in properties of elements across a period and down a group.
- Explain the periodicity of atomic radius, ionic radius, ionization energy, electron affinity and electronegativity.
Guidance
Tools and links
Definition
The atomic radius is defined as the distance from the centre of an atom to the outer edge. However, as atoms almost never exist as independent units, the measurement is taken as either the dispersion, or covalent radius, or the metallic radius.
If the element is in the solid form and the atoms are held together by dispersion forces, or covalent bonds only, as in the case of solid non-metals, then the radius is taken as one half the distance between the nuclear centres of neigbouring atoms.
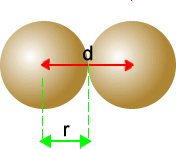
Measurement of atomic radius

The distance between the two nuclear centres is given by the red line, labelled 'd'.
The radius, 'r' is one half the value of the distance 'd'.
Similary for metals, the measurement is taken as one half the distance between nuclear centres in neighbouring atoms within a metallic lattice. While definitions are consistent, size comparison may be made between atoms or other particles.
Atomic radius
Atoms consist of electrons in shells surrounding a positive nucleus. There is attraction between the electrons and the nucleus and there is repulsion between the electrons themselves.
The radius of an atom is determined by two factors.
- The number of electrons shells - the greater the number of shells the larger the atom
- The number of protons in the nucleus - the greater the number of protons in the nucleus the smaller the atom.
The number of shells is the more important of the two factors, however across a period the number of shells does not change, it is the number of protons in the nucleus that affects the radius.
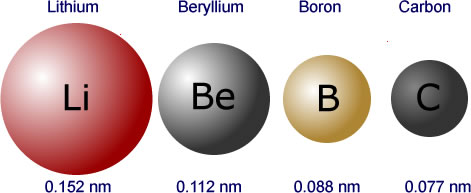 |
|
The atomic radii of the first four elements in period two shown to scale. This gives a good idea of just how much the increasing nuclear force acts on the outer energy shell. (scale 0.001 nm = 1 pixel) |
Ionization energy
"The energy required to remove 1 mole of electrons from 1 mole of gaseous atoms forming 1 mole of single charged ions".
M(g) → M+(g) + 1e-
The energy needed is dependent on the force with which the outer electron is held. This in turn is a function of the charge at the nucleus and the radius of the atom.
Electrostatic force is given by the equation:
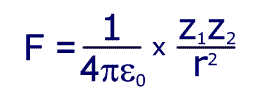
Where Z1 is the size (magnitude) if the charge on the nucleus, Z2 is the charge of an electron and 'r' is the distance between them.
Although this seems to be horribly complicated, the first section is just a constant and so the formula can be written in a much simpler way:
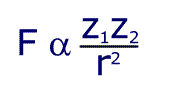
This shows that the force is proportional to the product of the charges, and inversely proportional to the square of the distance between them. Still confused? In simpler language, the further apart the charges, the weaker the force. The bigger the charge, the larger the force.
Summarised:
- Higher nuclear charge = stronger force.
- Smaller atomic radius = stronger force.
Down a group
Descending a group, the nuclear charge increases, but so does the atomic radius. These two factors would tend to cancel each other out, but the ionization energy decreases on descending a group. This is explained by inter-electron repulsions increasing as the number of shells increases. It is sometimes called the shielding effect.
Across a period
We have already seen that the nuclear charge increases across a period and the atomic radius decreases. We would expect a steady increase in the force needed to remove an electron (the ionisation).
However, when we graph the first ionization energies for the first 20 elements:

There is a general trend upwards across a period, but there are two points of inflexion (changes of direction) in both period 2 and 3. In period 2 the first point of inflexion is between element number 4 and 5, beryllium and boron.
There can be no doubt that the nuclear charge increases from beryllium to boron therefore the only possible reasons for boron requiring less energy to dislodge an electron are either inter-electron repulsion, or the electron is further from the nucleus that previously thought.
We now know that boron loses an electron from a 'p' sup-shell, which is more diffuse and further from the nucleus.
The point of inflexion between nitrogen and oxygen is explained by inter-electron repulsion between two electrons now paired in a 'p' orbital.
Electron affinity
The electron affinity is defined as the energy change when 1 mole of gaseous negative ions is formed from 1 mole of gaseous atoms and 1 mole of electrons. (definition not needed).
X(g) + 1e → X-(g)
The 1st electron affinity is usually exothermic (in some cases it is zero).
The electron affinity is a measure of the electron attracting ability of an atom. When the nuclear charge is high and the atomic radius is small the electron can approach closer to the atomic nucleus and feel a greater force if attraction. This translates into a larger energy release when the electron bonds to the atom.
Descending a group
The electron affinity decreases on descending a group. The number of energy shells increases and the electron is faced with more inter-electron repulsion as it approaches.
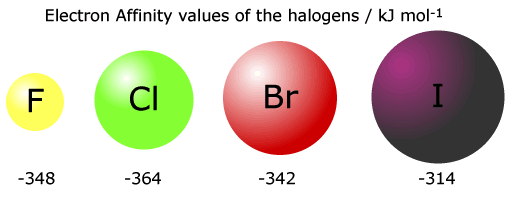
Notice that the 1st electron affinity of fluorine does not follow the group trend. This is thought to be because of inter-electron repusions in a full tightly packed second shell.
Across a period
The electron affinity increases across a period as the atomic radius gets smaller and the nuclear charge gets larger.
Electronegativity
The electronegativity of an element is a measure of its ability to attract electrons from within a covalent bond.
It is an artificial scale from 0.7 to 4.0, created by combining the ionization energy and the electron affinity of the elements. There are several versions of electronegativity although the most commonly used is that developed by Linus Pauling.
- The most electronegative element is fluorine, value = 4.0
- The least electronegative (sometimes called the most electropositive) is caesium, value = 0.7
Only elements with electronegativity values of 3.0 and above are considered electronegative.
Descending a group
The electronegativity decreases on descending a group. This makes common sense as electronegativity is derived from the ionization energy and electron affinity, which both decrease on descending a group.
Crossing a period
The electronegativity increases from left to right across a period, the most electronegative elements are nitrogen, oxygen and fluorine (in increasing order)
Comparing electronegativities
Information about the nature of chemical bonding can be predicted by looking at the electronegativities of the bonding atoms. The greater the difference in electronegativity the greater the polarisation of the bond.
Carbon has an electronegativity of 2.5 and hydrogen 2.1. These two values are relatively close to one another, so bonds formed between carbon and hydrogen are considered non-polar.
However, bonds formed between oxygen (3.5) and hydrogen (2.1) are highly polarised, (This is the basis behind intermolecular hydrogen bonding) whereas bonds formed between oxygen and elements with a lower electronegativity than hydrogen are polarised to such an extent that they are ionic.
In the same way the nature of the chlorides of the elements may be predicted.
Aluminium chloride formed from aluminium (electronegativity = 1.5) and chlorine (3.0)
AlCl3 falls right on the borderline of ionic/covalency. In fact, aluminium chloride is ionic at low temperatures, but covalent as the temperature rises. We consider it to be a covalent compound with highly polarised bonds. (the molecule itself is non-polar due to symmetry).
These 'rules' are not empirical in that they cannot be used mathematically. There is no 'magic' difference in electronegativity at which the change between ionic and covalent occurs, if this were the case then metal nitrides and sulfides would not be ionic. It is more a case of getting a feel for the concept and using the electronegativity values as a rough guide.
Worked examples
Q321-01 Which statement regarding the properties of elements arranged in the Periodic Table is correct?- Atomic sizes decrease going down a Group or Family.
- Atomic sizes increase going from Fr in Group 1, to F in Group 17.
- Atomic sizes decrease going from left to right in a Period.
- All atoms in the same Group have the same size.
|
Atomic sizes decrease going from left to right across a period, due to the increased nuclear charge acting on the same electron shell. |
Q321-02 In group 1, values of the metallic radii follow the order:
- Li > Na > K
- Na < K < Rb
- K > Rb > Cs
- Cs < K < Li
|
Metallic radius increases on descending a group due to the increasing number of electron shells, therefore Na < K < Rb |
Q321-03 A potassium atom has a larger atomic radius than a sodium atom. Which statement about potassium explains this difference?
- It has a larger nuclear charge
- It has a lower electronegativity
- It has more energy levels occupied by electrons
- It has a lower ionization energy
|
Potassium is in period 4, whereas sodium is in period 3, therefore potassium has more energy levels occupied by electrons |
Q321-04 Which of the following elements would be expected to have the largest atomic radius?
- Li
- Cs
- F
- I
|
Atomic radius increases from top right to bottom left, therefore Cs is expected to have the largest radius. |
Q321-05 Which atom has the smallest atomic radius?
- 19K
- 31Ga
- 35Br
- 37Rb
|
Atomic radius increases from top right to bottom left, therefore 35Br is expected to have the smallest radius. |
Q321-06 In general, atomic radius decreases according to which of the following patterns?
- within a group (family) from low to high atomic number.
- within a period from low to high atomic number.
- with an increase in the shielding of the nuclear charge.
- with an increase in the number of isotopes of an element.
|
Correct answer = within a period from low to high atomic number. |