Standard level
Oxides are binary compounds formed between elements and oxygen. They may be ionic or covalent, depending on the element. Chlorides are likewise binary compounds formed between elements and chlorine.
Syllabus ref: S3.1.5Structure 3.1.5 - Metallic and non-metallic properties show a continuum. This includes the trend from basic metal oxides through amphoteric to acidic non-metal oxides.
- Deduce equations for the reactions with water of the oxides of group 1 and group 2 metals, carbon and sulfur.
Guidance
- Include acid rain caused by gaseous non-metal oxides, and ocean acidification caused by increasing CO2 levels.
Tools and links
- Structure 2.1, Structure 2.2 - How do differences in bonding explain the differences in the properties of metal and non-metal oxides?
Period 3 oxides and chlorides
Oxygen is a highly reactive electronegative element that forms binary compounds easily. Chlorine is highly reactive also but it is less electronegative than oxygen and may form covalent compounds with metals of low electropositivity.
Moving from left to right across period three the elements change from metals to non-metals and the electronegativity increases. There is consequently a trend from ionic nature when combined with oxygen or chlorine towards covalent bonding. This gradual change is reflected in the melting points and boiling points of the period 3 oxides and chlorides.

Sodium oxide
Sodium is a highly electropositive element, which is reactive and burns easily in air or oxygen forming the oxide.
4Na + O2 → 2Na2O
Its oxide, sodium oxide, Na2O, is a white powder with a giant ionic structure. In keeping with giant ionic structures, it has a high melting and boiling point. When molten it is a good conductor of electricity, typical of an ionic compound.
Sodium chloride
Sodium chloride is a white crystalline ionic compound, formed by direct combination of sodium and chlorine gas. The sodium must be first warmed before immersion in chlorine. The reaction is highly exothermic.
2Na + Cl2 → 2NaCl
When molten, sodium chloride is a good conductor of electricity (electrolyte), typical of an ionic compound.

Magnesium oxide
Magnesium is an electropositive reactive metal which burns easily in air or oxygen forming magnesium oxide, MgO.
2Mg + O2 → 2MgO
Magnesium oxide is a white powdery compound with a giant ionic structure. In this case the giant ionic structure has double charged magnesium ions making its lattice relatively much stronger than that of sodium oxide. When molten it is a good conductor of electricity, typical of an ionic compound.
Magnesium chloride
Magnesium chloride is a white crystalline ionic solid that can be formed by burning magnesium in chlorine. Once again, it is an exothermic reaction.
Mg + Cl2 → MgCl2
When molten, it is a good conductor of electricity, typical of an ionic compound.

Aluminium oxide
Aluminium is a poor metal from group 13, but will burn in air at high temperatures, or when powdered, to produce aluminium oxide. The oxide has a giant ionic structure with 3+ charged aluminium ions making the lattice extremely strong.
4Al + 3O2 → 2Al2O3
It has a very high melting point and boiling point. When molten it is a good conductor of electricity, typical of an ionic compound. It is used in the extraction of aluminium by means of electrolysis. (Hall Cell)
Aluminium chloride
Aluminium chloride can be formed in the anhydrous state by heating aluminium in a stream of chlorine gas. Aluminium chloride is covalent under these conditions and sublimes from the reaction vessel and may be condensed and collected by cooling.
2Al + 3Cl2 → 2AlCl3
Aluminium chloride is covalent not ionic as would normally be expected from a metal and a non-metal binary compound.
Aluminium is electron deficient in the structure AlCl3 and for this reason dimerises to form Al2Cl6, in which a lone pair from a chlorine on each aluminium donates into the opposite electron deficient aluminium atom.
It does not conduct electricity when in the liquid state. This supports its covalent nature.

Silicon dioxide
Silicon is a non-metal and both it and its oxide have giant covalent structures.
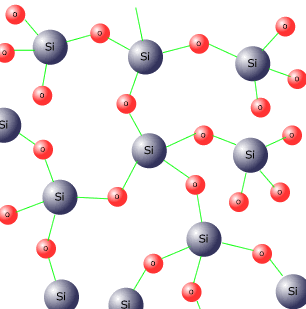
The structure of SiO2 is similiar to diamond only with oxygen atoms bridging the gaps between the tetrahedral silicon atoms.
Although it seems difficult to ascertain the formula from such a mass of atoms, if you consider that every oxygen is attached to two silicon atoms and every silicon atom is attached to four oxygen atoms, the average is one silicon for every two oxygen atoms, i.e. SiO2
Giant covalent structures are the hardest and strongest known, hence silicon dioxide has an extremely high melting and boiling point. It does not conduct electricity when in the liquid state. This supports its covalent nature.
Silicon chloride
Silicon chloride is a colourless covalent liquid formed by directly heating silicon in a stream of chlorine gas at high temperature.
Si + 2Cl2 → SiCl4
It does not conduct electricity when in the liquid state. This supports its covalent nature.

Phosphorous oxides
Phosphorus burns readily in air forming two distinct oxides, depending on the amount of air, or oxygen, available.
4P + 3O2 → P4O6
4P + 5O2 → P4O10
Both of the oxides have simple covalent structures with low melting and boiling points. The structures consist of pyramids of phosphorus atoms linked together by bridging oxygens. In the case of phosphorus(V) oxide, there are also oxygen atoms coodinated by the lone pairs of the phosphorus atoms. They are a liquid and solid respectively at room temperature.
Neither oxide conducts electricity when in the liquid state. They are covalent in nature.

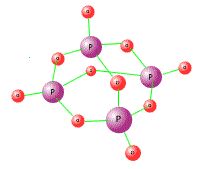
Phosphorous chlorides
Phosphorus forms two chlorides when directly heated in chlorine gas.
2P + 3Cl2 → 2PCl3
PCl3 + Cl2 ⇋ PCl5
The second reaction is an equilibrium that has been studied extensively and, as such, often appears in equilibrium-type questions.
Both compounds are covalent, with low melting and boiling points.
Neither chloride conducts electricity when in the liquid state. They are covalent substances.

Sulfur oxides
Sulfur is a yellow crystalline element that burns readily in air forming an oxide, sulfur dioxide (sulfur(IV) oxide). A second oxide, sulfur trioxide (sulfur(VI) oxide) can also be formed in excess air in the presence of a catalyst at 450ºC.
S + O2 → SO2
2SO2 + O2 → 2SO3
Both oxides are simple covalent compounds with low m.p. and b.p. sulfur dioxide (sulfur(IV) oxide) is a gas at room temperature.
Neither oxide conducts electricity when in the liquid state. They are covalent substances.

Chlorine oxides
Chlorine is a light green poisonous gas with a familiar smell of 'swimming pools'. It forms a series of oxides, the simplest of which is chlorine (I) oxide with the formula Cl2O, which has a simple covalent structure with a low melting and boiling point.
Its higher oxide, chlorine heptoxide Cl2O7, is a covalent substance with a low melting point. It is an insulator in the liquid state.
Summary
Bonding in the oxides and chlorides of the third period
Worked examples
Q526-01 Which of the following statements is/are correct- I. The melting points decrease from Li to Cs for the alkali metals
- II. The melting points increase from F to I for the halogens
- III. The melting points decrease from Na to Ar for the period 3 elements
- I, II and III
- II and III only
- I and III only
- I and II only
Correct response = I and II only |
Q526-02 Why do the boiling points of the halogens increase down the group?
- There is an increase in bond enthalpy
- There is an increase in bond polarity
- There is an increase in the strength of temporary dipoles
- There is an increase in electronegativity
|
Dispersion forces are also called temporary dipoles. These increase with increasing relative mass. |
Q526-03 Which statement is correct for a periodic trend?
- ionization energy increases from Li to Cs
- Melting point increases from Li to Cs
- ionization energy increases from F to I
- Melting point increases from F to I
|
Correct response = Melting point increases from F to I |
Q526-04 Which properties are typical of most non-metals in period 3 (Na to Ar)
- I. They form ions by gaining one or more electrons
- II. They are poor conductors of heat and electricity
- III. They have high melting points
- I and II only
- I and III only
- II and III only
- I, II and III
|
Non-metals in period three include Si, P, S, Cl and Ar. Most of these form ions by gaining electrons and are poor conductors of electricity. They do not have high melting points due to simple covalent bonding (except Si and Ar), therefore correct response = I and II only |
Q526-05 Which property decreases down group 17 in the periodic table?
- Atomic radius
- Electronegativity
- Ionic radius
- Melting point
|
Group 17, the halogens become less electronegative descending the group. |
Q526-06 Which of the physical properties below decrease with increasing atomic number for both alkali metals and the halogens?
- I. Atomic radius
- II. ionization energy
- III. Melting point
- I and II only
- I and III only
- II only
- I, II and III
Correct response = II only |
Q526-07 Which of the following oxides is (are) gases at room temperature?
- I. SiO2
- II. P4O6
- III. SO2
- I only
- III only
- I and II only
- II and III only
Correct response = III only |
Q526-08 Which of the following properties of the halogens increase from F to I?
- I. Atomic radius
- II. Melting point
- III. Electronegativity
- I only
- I and II only
- I and III only
- I, II and III
Correct response = I and II only |
Q526-09 Which property increases with increasing atomic number for both the alkali metals and the halogens?
- Atomic radius
- Electronegativity
- ionization energy
- Melting point
|
Q526-10 At standard temperature and pressure (STP), chlorine is
- a white, unstable solid.
- a brown liquid
- a soft, reactive solid.
- a green gas.
|
Fluorine is a pale yellow gas at STP, Chlorine is a green gas at STP. Bromine is a dark red liquid, Iodine is a grey, shiny, crystalline solid |