Higher-level only
Nuclear magnetic resonance relies on the magnetic field produced by a spinning nucleus containing an odd number of nucleons (protons or neutrons). In the presence of an external magnetic field the nucleus can exhibit more than one spin state and can move between these states by the absorption of electromagnetic radiation of a specific frequency (energy).
The energy absorbed can be detected and from this information about the location (environment) of the nucleus can be deduced.
NMR is probably the most useful tool in the organic chemists arsenal for structural determination.
As organisms are mainly water (containing H atoms with an odd number of nucleons), NMR has developed into an invaluable medical diagtnostic tool, called an MRI (magnetic resonance instrument) scan.
Syllabus ref: S3.2.11Structure 3.2.11 - Individual signals can be split into clusters of peaks. (HL)
- Interpret 1H NMR spectra from splitting patterns showing singlets, doublets, triplets and quartets to deduce greater structural detail.
Guidance
- Data for interpretation of 1H NMR spectra are given in the data booklet.
Tools and links
Spin-spin coupling
Spin-spin coupling refers to the influence that hydrogen nuclei have on other neighbouring hydrogen nuclei. It is spin-spin coupling that produces splitting patterns.
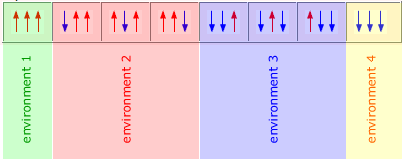
Initially, the spinning hydrogen nucleus is under the influence of an external and local magnetic field. This means that its two magnetic spin states are separated by a specific energy difference. A neighbouring spinning hydrogen nucleus also provides a local magnetic field that either adds to the field experienced by the first nucleus or subtracts from it.
Hence, protons with a neighbouring proton show two distinct signals, one representing the additive influence of its neighbour and the other representing the subtractive influence.
The proton spin is assigned quantum values of +1/2 or -1/2 and each of these two states produces a slighty different magnetic field. This means that neighbouring protons experience two different fields, one when the neighbouring hydrogen is in the +1/2 state and the other when the neighbouring hydrogen is in the -1/2 spin state.
However, it is often the case that there is more than one neighbouring hydrogen atom, in which case the number of possible states that a hydrogen can experience is equal to all of the different possible arrangement of spin states created.
For example, a CH2 group contains two hydrogen atoms, each of which can have two possible spin states, +1/2 and -1/2. Therefore, the number of possible arrangements of states for the two hydrogens is:
| state 1 | state 2 | state 3 | state 4 |
| +1/2 and +1/2 | -1/2 and +1/2 | +1/2 and -1/2 | -1/2 and -1/2 |
However, as these two hydrogen atoms are identical and in equivalent environments, then a neighbouring hydrogen atom is not able to distinguish between states 2 and 3, meaning that there are only three different states, although the -1/2 and +1/2 states have twice the probability.
This is reflected in the signal formed by a hydrogen atoms adjacent to this CH2 group. It's signal is split into three peaks, the middle one of which has double the size (as its probability is twice as great).
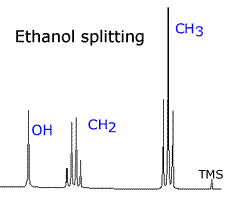
|
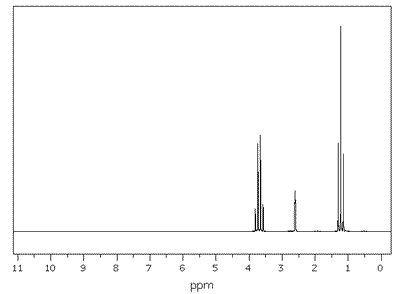
In the NMR spectrum of ethanol, the CH3 protons have two neighbouring hydrogen atoms. These neighbours have three possible combined spin states, providing three possible magnetic environments for the CH3 group. Hence, the CH3 group is split into a triplet by the neigbouring CH2 group. Likewise, the CH2 group is split into a quartet by its CH3 neighbours, which provide four different environments.
|
| ethanol: CH3 - CH2 - OH | Interestingly, the OH, hydrogen has little effect in terms of splitting. It hardly splits or gets split. |
Splitting patterns
The splitting patterns in a signal caused by neighbouring protons are given names according to the number of peaks in the pattern.
The splitting is caused by the spin states of the neighbouring hydrogen nuclei, and as explained in the previous section, some of the combinations of spin states are more probable than others. This means that the intensities of the individual peaks within a splitting patterns follows predictable patterns.
Clearly, there is no patterns to be expected from a singlet - there is only one peak. However, a doublet is caused by the two possible spin states of a single neighbouring proton. Both of these states are equally likely and so the intensity of both peaks is the same.
However, with two neighbouring protons there are three possible environments leading to the triplet and the intensity of the central peak is double that of the outer peaks.
In the case of a quartet, the peak intensities are in a ratio of 1:3:3:1.
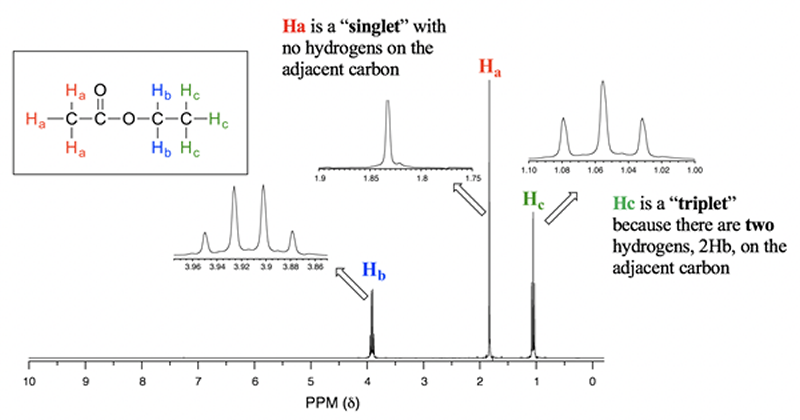
Multiplets
As carbon can only bond to four atoms in total, multiplets must be formed by more than one set of neighbouring protons on two, or more adjacent carbon atoms.
If the carbon environments are identical, this can lead to symmetrical multiplets of greater than four peaks, for example (CH3)2CH-.
We would expect the CH proton to give a signal with a symmetrical multiplet of seven peaks caused by the two adjacent methyl groups.
More commonly, multiplets are caused by having neighbouring protons on either side of the proton causing the signal. If the neighbouring protons are in different environments, then they will split the signal proton by different amounts causing a mess due to double splitting.
This is the case for compounds with phenyl groups, C6H5- where the protons are all splitting each other by slightly different amounts, due to the group attached to the benzene ring. A characteristic messy multiplet appears on the spectrum at about delta 7.
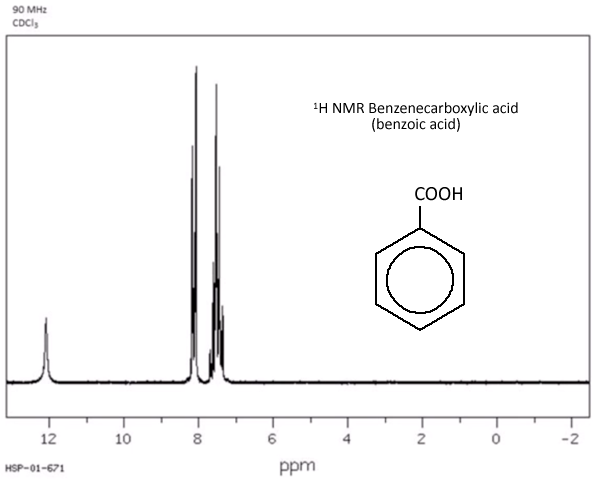
Worked examples
Q1135-01 How many peaks are there in the 1H NMR spectrum of (CH3)2CHCOCH3- 2
- 3
- 4
- 5
|
There are three different hydrogen environments, and hence three peaks. |
Q1135-02 How many lines are present in the 1H NMR spectrum of C(CH3)4?
- 1
- 3
- 4
- 12
|
There is only one hydrogen environment, and hence one peak. |
Q1135-03 Which of the following compounds exhibits three lines in the 1H NMR spectrum?
- I- CH3CH2OCH3
- II- (CH3)3CCl
- III- CH3CH2COOH
- I only
- II only
- I and III only
- I, II and III
|
Compounds I and III have three different hydrogen environments, and hence three signals. |
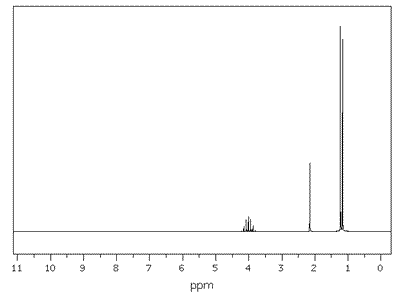
Q1135-04 Which of the compounds below corresponds to the NMR spectrum shown?
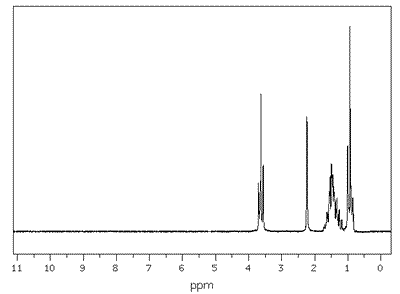
- (CH3)4C
- CH3COOCH3
- CH3CH2CH2OH
- CH3CH2CH2CH2OH
|
Only compound D has four different hydrogen environments. This matches the four separate signals in the spectrum. |
Q1135-05 How many lines would be expected in the proton NMR spectrum of benzene C6H6?
- 1
- 2
- 6
- 42
|
All of the hydrogen atoms are in the same environment and will appear as a single line |
Q1135-06 How many peaks are there is the 1H NMR spectrum of ethanol
- 2
- 3
- 6
- 5
|
There are three different hydrogen environments and hence, three distinct separate peaks (they are, however, split by neighbouring protons).
|
Q1135-07 Which compound shows three different environments for hydrogen atoms in the 1H NMR spectrum.
- CH3CH2CH3
- CH2OHCH2OH
- CH3CH2CH2OH
- CH3CH(OH)CH3
|
Compound A has only two different hydrogen environments. Compound B also has two different hydrogen environments. Compound C has four different hydrogen environments. Therefore the answer is propan-2-ol, D.
|
Q1135-08 Which isomer of C2H6O2 has a 1H NMR spectrum with a different number of peaks from the others?
- HOOCH2CH3
- CH3COOCH3
- CH3OCH2CHO
- CH3CH2COOH
|
Compound B, methyl ethanoate, has only two peaks, due to two different hydrogen environments.
|
Q1135-09 How many different environments for hydrogen atoms are present in the 1H NMR spectrum of the following compound?
(CH3)2CHCH2CH3
- 3
- 4
- 5
- 9
|
The compound is methylbutane and has four different hydrogen environments. It is, in practice, difficult to see the separate peaks as they are all very close together. There are no electronegative atoms or groups to shift the signals downfield.
|
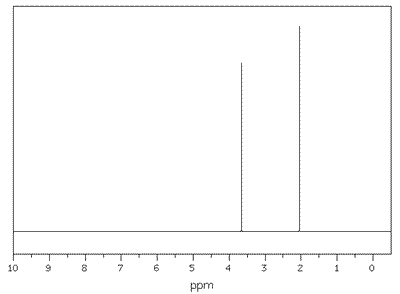
Q1135-10 Which compound gives two distinct peaks in the 1H NMR spectrum?
- C6H6
- C2H5OH
- (CH3)3COH
- CH3OCH3
|
Compound C, methylpropan-2-ol, has only two different hydrogen environments. One due to the three methyl groups (9 hydrogen atoms) and the other due to the alcohol hydrogen (1 hydrogen). Neither of these signals is split.
|