Higher-level only
This is a sub-set of isomerism that is due to the positions of the atoms in space. There are two forms of stereoisomers, geometric and optical. The term "geometric" has largely been superceded by "cis-trans" isomerism and is caused by the prevention of rotation about carbon-carbon bonds. Optical isomerism is caused by the asymmetry of a molecule having non-superimposible mirror image molecules.
Syllabus ref: S3.2.7Structure 3.2.7 - Stereoisomers have the same constitution (atom identities, connectivities and bond multiplicities) but different spatial arrangements of atoms. (HL)
- Describe and explain the features that give rise to cis-trans isomerism; recognize it in non-cyclic alkenes and C3 and C4 cycloalkanes.
- Draw stereochemical formulas showing the tetrahedral arrangement around a chiral carbon.
- Describe and explain a chiral carbon atom giving rise to stereoisomers with different optical properties.
- Recognize a pair of enantiomers as non-superimposable mirror images from 3D modelling (real or virtual).
Guidance
- Nomenclature using the E‐Z system will not be assessed.
- The terms “chiral”, “optical activity”, “enantiomer” and “racemic” mixture should be understood.
- Knowledge of the different chemical properties of enantiomers can be limited to the fact that they behave differently in chiral environments.
- Wedge-dash type representations involving tapered bonds should be used for the representation of enantiomers.
Tools and links
Stereoisomerism
'Stereo' - from two positions in space.
This is when two molecules have the same molecular formula and the same groups of atoms, but they are arranged in such a way that different forms of the molecule are non-superimposible.
There are two types of stereoisomerism:
- Cis-trans or E/Z isomerism
- Optical isomerism.
Stereoisomers have the same molecular formula and the same systematic name, therefore extra pre-prefixes must be used to differentiate the structures.
Cis-trans isomerism
Cis-trans isomerism is caused by the molecule having the same basic structure, but a different geometry (don't let the word put you off, it's not difficult).
This can happen when groups, or atoms, are fixed on one side of a carbon chain by a lack of rotation between two carbon atoms in the chain, usually caused by a double bond.
Isomers are named by using the prefix 'cis' to indicate that important groups are on the same side of the chain, or 'trans' to indicate that the important groups or atoms lie on opposite sides of the carbon chain.
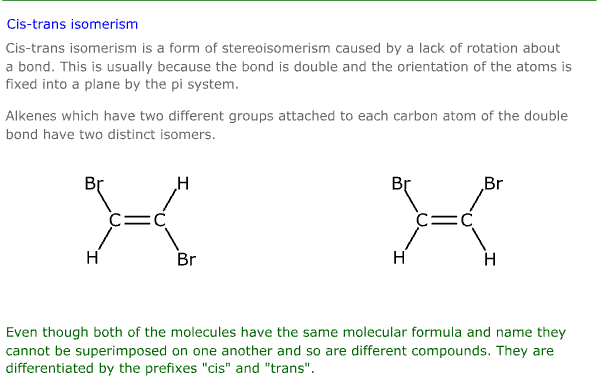
In the diagram above, the 'cis' isomer (on the right) has two bromine atoms which lie on the same side of the carbon chain, (the top, as we look at it), while the 'trans' isomer has the two bromine atoms on opposite sides of the carbon chain.
The relationship between the atoms within each structure is unique, giving rise to different chemical and physical properties. This is true for all geometric isomers.
Alkenes
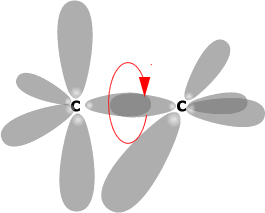
The most important group of compounds that exhibit geometric isomerism is the alkenes. There is no rotation about a double bond due to the nature of the 'pi' bond system, which would break if the molecule were to rotate about the carbon - carbon bond.
 |
 |
|
The 'pi' molecular orbital system arises by lateral
overlap of parallel 'p' orbitals.
|
If rotation happens about the C-C (sigma bond) then
the 'p' orbitals can no longer overlap.
|
Cyclic molecules

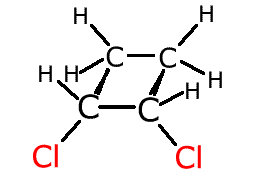
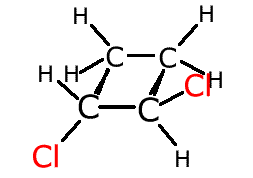
Other systems with restricted rotation are cyclic molecules. If rotation were to be attempted about a carbon-carbon bond it would break the molecule. This also gives rise to geometric isomerism.
In the cyclobutane molecule above, the hydrogen atoms at the front (red) are both below the plane of the ring. They are fixed in this position by a lack of rotation between the two carbon atoms at the front.
If two different groups replace these two red hydrogen atoms this gives rise to geometric isomerism.
If the different atoms cross from under the ring plane to over the ring plane the molecule is referred to as 'trans'. If the different attachments both lie on the same side of the ring plane they are referred to as 'cis'.
|
cis-1,2-dichlorocyclobutane
|
trans-1,2-dichlorocyclobutane
|
 |
 |
Physical properties
Geometric isomers differ in density, viscocity, refractive index, enthalpy of vaporisation and they also have different melting and boiling points. The arrangement of the atoms in space generates varying degrees of intermolecular attractions, giving rise to differences in the physical properties.
|
cis-but-2-ene
|
trans-but-2-ene
|
|
|
m.p./K
|
134
|
167
|
|
b.p./K
|
277
|
274
|
|
ΔH(vap)
|
22.7 kJ mol-1
|
22.0 kJ mol-1
|
Chemical properties
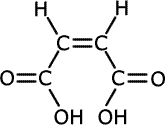
The double bond holds groups in specific orientations, having an effect on the chemical properties of geometric isomers. The classic case is that of the butenedioic acids, HOOCCH=CHCOOH. They have the following structures:
 |
 |
|
cis-butenedioic acid
|
trans-butenedioic acid
|
In the cis-isomer, the double bond holds the two carboxylic acid groups close together and in the same plane, whereas in the trans-isomer they are far apart. Heating the two isomers separately has very different effects.
The cis-isomer easily loses water (dehydrates) to give a species known as an anhydride, specifically butenedioic anhydride.
 |
140ºC
→ moderate heat |
 |
|
cis-butenedioic acid
|
butenedioic anhydride
|
For this reaction to occur, the two carboxylic acid groups must be close together. They are not close enough in the trans-isomer, so the reaction cannot happen.
Nitrogen compounds
Nitrogen also exhibits stereoisomerism in compounds in which the nitrogen atom is double bonded. In this case the lone pair on the terminal nitrogen can be considered an attached group.
|
Example: The compound H-N=N-H has two cis-trans isomers. In one of the isomers the two hydrogens attached to the two nitrogens are on the same side of the double bond. This is the cis-isomer. In the other geometric isomer the two hydrogens 'cross' the double bond; the trans-isomer. |
Optical isomerism
Optical isomers have an identical arrangement of atoms, but the isomers are mirror images of one another. These mirror images are non-superimposible, just like a pair of gloves. This can only happen if the molecule is asymmetrical. The easiest way for a molecule to be asymmetrical is to have a carbon atom with four different groups or atoms attached. Such an asymmetric carbon atom is called a chiral atom, or centre of chirality.
Mirror image optical isomers are called enantiomers.
Physical properties of enantiomers
Optical isomers possess identical physical properties (except in some cases
they form asymmetric mirror image crystals), with one important exception;
a pure optical isomer, or solution of a pure optical isomer rotates the plane
of plane polarised light through an angle. The angle of rotation depends on
the nature of the optical isomer, the solution strength and path length.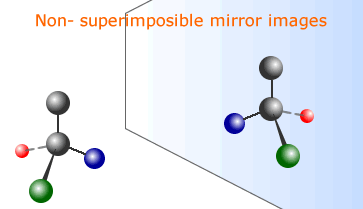
Each optical isomer rotates the plane of polarised light by the same amount, but in the opposite sense, i.e. if one enantiomer rotates polarised light clockwise, then the other enantiomer will rotate plane polarised light through the same angle anticlockwise.
Equal concentrations of two enantiomers do not rotate plane polarised light, as the opposite effects of each optical isomer cancel each other out. This is known as external compensation. Such an externally compensated solution, containing equimolar amounts of two enantiomers, is known as a racemic mixture, or racemate.
Although optical isomerism may seem at first sight to be rather a trivial form of isomerism, it is extremely important in biological systems. The chemical properties of optical isomers are identical EXCEPT when reacting with other optical isomers. This is the basis of the 'key and lock' activation of organic compounds by enzymes.
Biological systems contain many proteins and complex aminoacids, which are optically active. They may be stimulated to react in certain ways by optically active enzymes (biological catalysts).
Chemical properties of enantiomers
The relative positions of all of the component atoms in a pair of optical
isomers are identical. This means that in almost every situation the optical
isomers have identical chemical properties. However, there is one situation
in which two optical isomers can have different reactivity, including one
being totally unreactive while the other is reactive, and this is when they
are reacting with another molecule at a site which itself is asymmetric and
has optical isomers.
The simplest way to appreciate this is to consider the two optical isomers to be a pair of hands, one left hand and one right hand. The reagent which they wish to react with is a left glove. Clearly, the left hand can fit into the left glove, while the right hand cannot.
This principle occurs regularly in biological systems. Many enzymes are chiral and many of the active sites that they bind to are also chiral. The type of optical isomer, + or -, becomes very important. This is sometimes referred to as the lock and key principle. The enzyme is the key and the protein with which it is going to react is the keyhole of the lock. If the key is incorrect it cannot open the lock.
Measuring optical rotation
The 'optical rotation' means the angle through which plane polarised light is turned by the optical isomer under investigation. It is measured using a polarimeter.
An optical isomer may rotate plane polarised light in a clockwise manner (+) or in an anticlockwise manner (-). The isomers are said to be dextrorotatory (rotate to the right) and Laevorotatory (cause rotation to the left). There is no way of knowing how a specific optical isomer will affect plane polarised light just by looking at the structure.
|
|
|
The specific rotation of a chemical compound is defined as the observed angle of optical rotation a when plane-polarised light is passed through a sample with a path length of 10 cm at a sample concentration of 1 g/100cm3.
Classifying enantiomers
There are several systems for classifying the two enantiomeric forms arising from an asymmetric centre. They either depend on the effect that the enantiomer has on plane polarised light, the configuration with respect to a reference molecule or the configuration with respect to a system of priorities Cahn-Ingold-Prelog
The d/l system
An enantiomer can be named by the direction in which it rotates the plane of polarized light.
If it rotates the light clockwise (as seen by a viewer towards whom the light is traveling), that enantiomer is labeled (+). Its mirror-image is labeled (-).
The (+) and (-) isomers have also been termed d- and l-, respectively (for dextrorotatory and levorotatory). Naming with d- and l- is easy to confuse with D- and L- labeling and is therefore strongly discouraged by IUPAC.
The D/L system
An optical isomer can be named by the spatial configuration of its atoms. The D/L system (named after Latin dexter and laevus, right and left), not to be confused with the d- and l-system, does this by relating the molecule to glyceraldehyde.
To determine the D/L isomeric form of an amino acid the "CORN" rule is used. The groups:
COOH, R, NH2 and H (where R is the side-chain)
are arranged around the chiral center carbon atom. With the hydrogen atom away from the viewer, if the arrangement of the CO, R & N groups around the carbon atom is counter-clockwise, then it is the L form.
If the arrangement is clockwise, it is the D form. The L form is the usual one found in natural proteins. For most amino acids, the L form corresponds to an S absolute stereochemistry, but is R instead for certain side-chains.
The R/S system
The R/S system is the most important nomenclature system for denoting enantiomers, which does not involve a reference molecule such as glyceraldehyde.
It labels each chiral center R or S according to a system by which its substituents are each assigned a priority, according to the Cahn–Ingold–Prelog priority rules (CIP), based on atomic number.
If the center is oriented so that the lowest-priority of the four is pointed away from a viewer, the viewer will then see two possibilities:
If the priority of the remaining three substituents decreases in clockwise direction, it is labeled R (for Rectus, Latin for straight), if it decreases in counterclockwise direction, it is S (for Sinister, Latin for left).
Worked examples
Q1017-01 Does the following molecule exhibit cis-trans isomerism?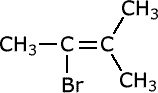
|
For cis-trans isomerism to be possible there must be two different groups on BOTH carbons either side of the double bond (or restricted rotation bond). There is no cis-trans isomerism possible. |
Q1017-03 Which of the following is different for the two isomers cis-1,2-dibromoethene and trans-1,2-dibromoethene?
- Relative molecular mass
- Boiling point
- Molecular formula
- Molar mass
|
Of the choices, only the boiling point is different for two cis-trans isomers. |
Q1017-04 Which of the following is the same for two cis-trans isomers?
- Viscocity
- Density
- Boiling point
- Molecular formula
|
Cis-trans isomers, in keeping with all isomers, have the same molecular formula. |
Q1017-05 How many different isomers are possible for dibromocyclobutane?
Answer
|
There are five possible isomers:
|
Q1017-06 For which of the following compounds are cis-trans isomers possible?
- I. CH3CH=CH2
- II. CH3CH=CHCl
- III. CHCl=CHBr
|
In compound I there are two groups the same on one of the carbon atoms, so no cis-trans isomerism is possible. Correct response, II and III |
Q1017-07 Which one of the following molecules can exist as cis-trans isomers?
- (CH3)2C=CHCH3
- CH3CH2CH=CHCH3
- H2C=CHCH2CH2CH3
- CH3CH(CH3)CH=CH2
|
Only compound B has two different groups on BOTH carbons attached by the double bond. |
Q1017-08 Which of the following compounds exhibit cis-trans isomerism?
- CH2=NH
- CH3N=NH
- (CH3)2C-NH2
- (CH3)3C-NHCH3
|
For nitrogen to exibit cis-trans isomerism there must be two different groups attached to both sides of the double bond (for nitrogen the lone pair counts as a group). Molecules C and D don't have a double bond and so are excluded. Molecule B has two separate groups attached to either side of the double bond and DOES exhibit cis-trans isomerism. |
Q1017-09 Which of the following compounds do not exhibit cis-trans isomerism:
- HN=NCH3
- H2NCH=CHNH2
- H2N-N=NH2
- H2N-N=CHNH2
|
Compound C has two hydrogen atoms on one side of the double bond and cannot have cis-trans isomers. |
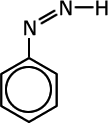
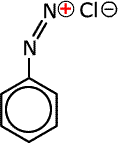
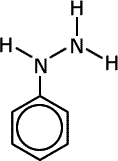
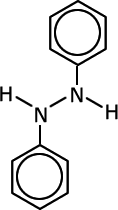
Q1017-10 Which of the following compounds can exhibit cis-trans isomerism:
|
A
|
B
|
C
|
D
|
 |
 |
 |
 |
|
Compounds C and D do not have a double bond and are excluded. The diazonium ion, B, has only one lone pair on the nitrogen carrying the charge and no other group. This lone pair will orientate in a linear fashion to the double bond to minimise repulsion. Compound A has cis-trans isomers |
