Self-assessment tests and teaching resources
The following tests can be set for a specific number of questions and the results will be posted to your email. An analysis of incorrect responses is included.
| Description | |
| Stoichiometry | All areas of the quantitative chemistry topic. |
| Sto01 | Relative mass |
| Sto02 | Oxidation state |
| Sto03 | Moles |
| Sto04 | Avogadro's number |
| Sto05 | Moles and particles |
| Sto06 | Formulation |
| Sto07 | Empirical formula |
| Sto08 | Mass and moles relationships |
| Sto09 | Reacting mass and moles |
| Sto10 | Reaction types |
| Sto12 | Reacting particles in chemical reactions |
| Sto13 | Conservation of mass in chemical reactions |
| Sto14 | Definitions |
| Sto15 | Experimental data |
| Sto16 | Determination - relative mass of active metals |
| Sto17 | Equations |
| Sto18 | Balancing coefficients in equations |
| Sto19 | Reacting particles in balanced equations |
| Sto20 | Conservation of mass in reactions |
| Sto21 | Avogadro's law and gas volumes |
| Sto32 | Calculating solution concentration |
| Sto33 | Solution preparation by dissolution and dilution |
| Sto34 | Reactions in solution |
| Sto35 | Acid - base titrations |
| Sto36 | Back-titration |
| Sto39 | Experimental and theoretical yield in chemical reactions |
| Sto40 | Experimental inaccuracies |
| Teaching resources | |
| STR01 | Classification of matter |
| STR02 | Mole concept |
| STR03 | Solution preparation |
| STR04 | Titration |
| STR05 | Heating magnesium |
Internal video links
IB Chemistry syllabus videos (first exams May 2025), by Colourful Solutions.
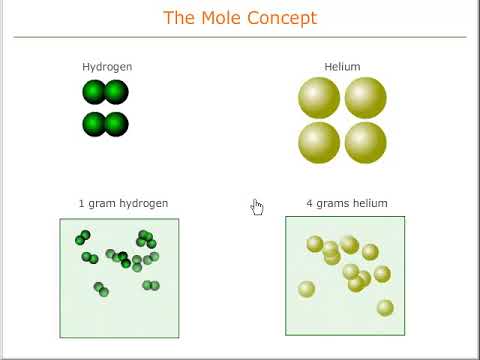
Relative mass and the mole concept
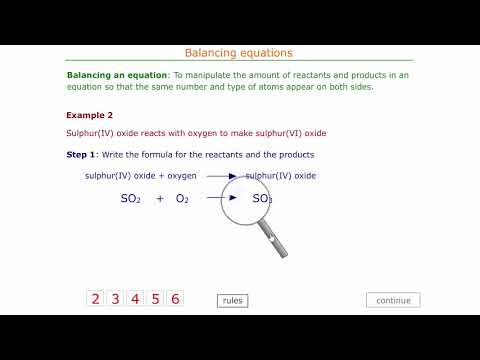
Balancing chemical equations
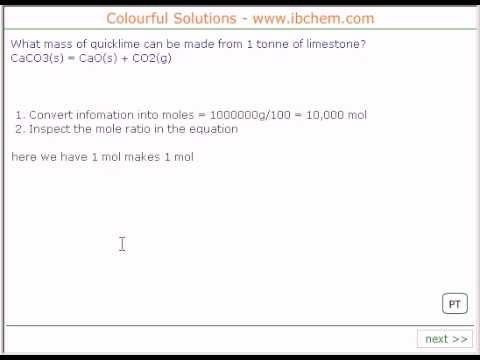
Mass to mass calculations
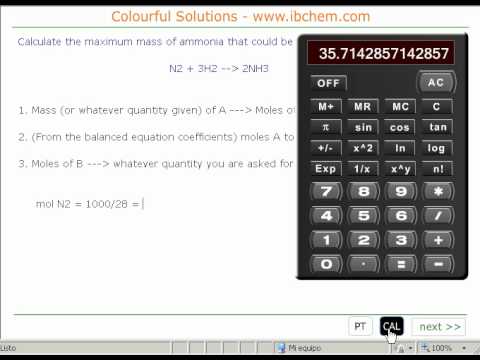
Mass to mass calculations in chemical equations
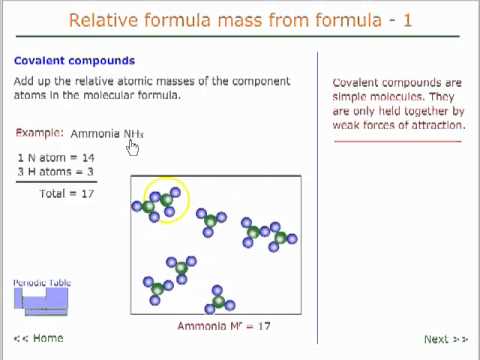
Relative formula mass 1 - covalent compounds
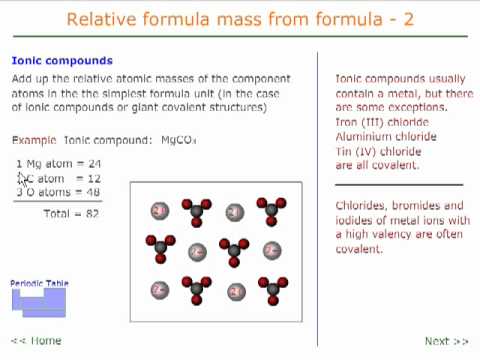
Relative formula mass 2 - ionic compounds
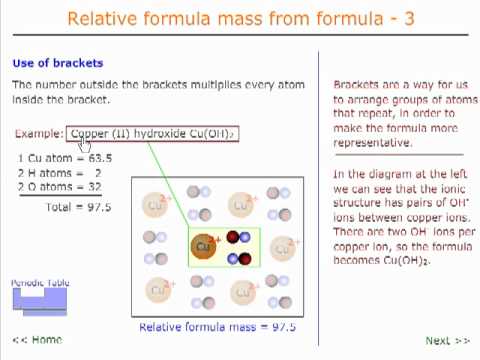
Relative formula mass 3 - formulae with brackets
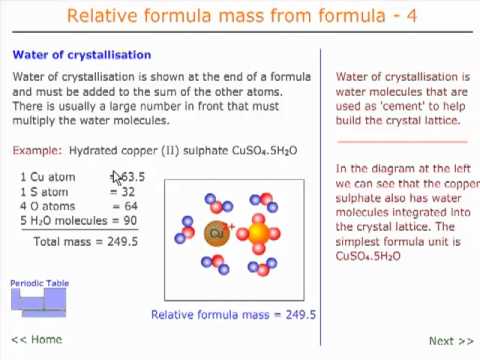
Relative formula mass 4 - water of crystallisation
External video links
The following videos have been prepared for the IB Chemistry syllabus (first exams May 2025), by Richard Thornley, Mike Sugiyama Jones and others.

Atoms, elements, compounds and mixtures

Identifying atoms, elements, compounds and mixtures

The kinetic theory of matter

State symbols

Changes of state
Temperature and kinetic energy

Perfect crystals

The mole concept
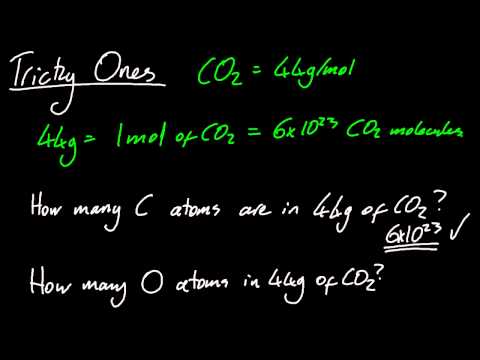
The number of particles in a mole
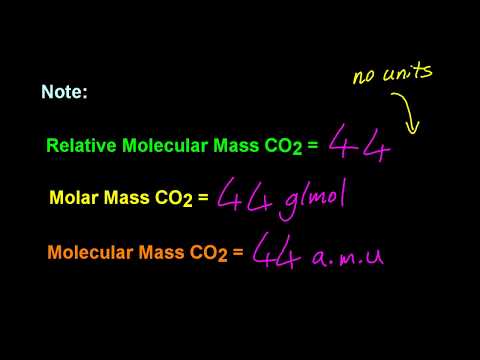
Relative mass
Molar mass

Solving moles problems
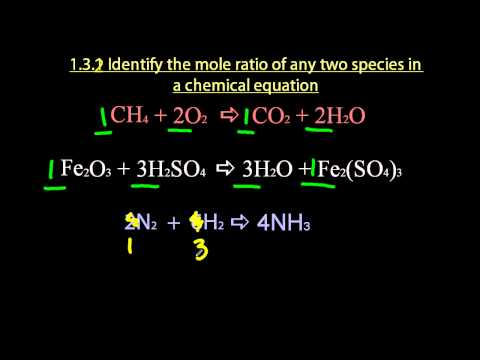
Identifying mole ratios
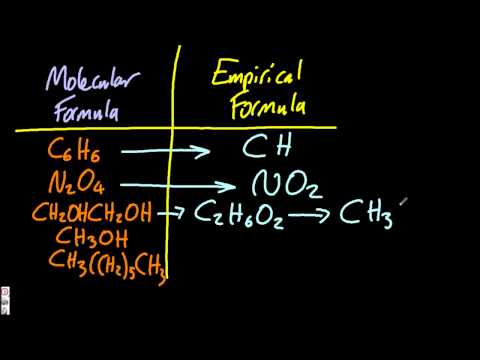
Empirical and molecular formula
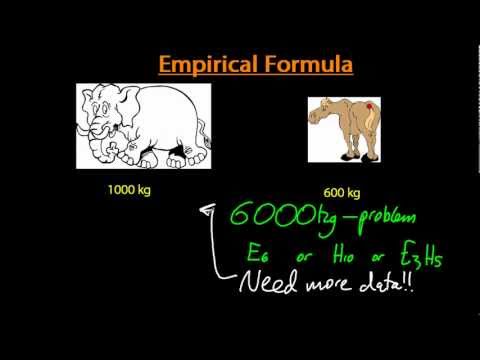
Empirical formula and percentage composition
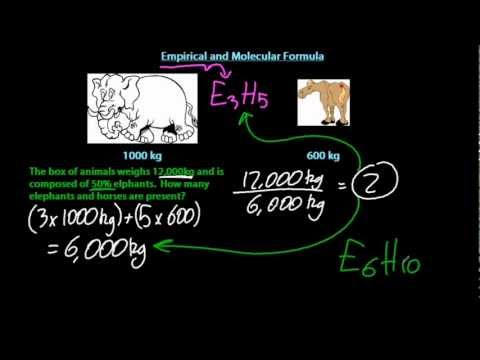
Determining molecular formula from percentage data

Determining molecular formula from combustion data

Solving solutions problems

Distinguish between solution, solvent and solute

Converting concentrations

Avogadro's law of gas volumes