|
'Poly' = many, therefore polyatomic = many atoms. |
|
Single ions are stable due to the completed outer shell. Groups of atoms, covalently bonded together, can also produce stable structures by addition, or removal of electrons, to create groups of atoms in which all of the atoms have full outer shells and the whole structure carries an electrical charge. Such arrangements are called polyatomic ions.
Polyatomic ions behave very much like simple (monoatomic ions), in that they form compounds, which are giant structures of repeating ionic units (lattices), with metal ions or other polyatomic, oppositely charged ions.
You must be familiar with several of these ions, their formulas, charges and structures
Common polyatomic negative ions (anions)
Students should be familiar with the following polyatomic ions: ammonium, hydroxide, nitrate, hydrogencarbonate, carbonate, sulfate and phosphate.
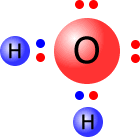
The simplest of the polyatomic ions is the hydroxide ion. This can be thought of as a water molecule that has lost a hydrogen ion (proton).
 |
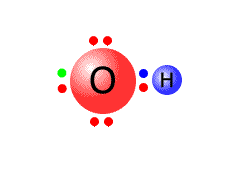 |
| The water molecule has four pairs of electrons of which only two are used to bond hydrogen atoms, whereas the hydroxide ion has now lost a hydrogen ion, leaving behind the pair of electrons. All of the outer shells are full - the ion has a single negative charge (shown in green). | |
The sulfate ion comes from sulfuric acid reacting with bases and active metals. It has four pairs of electrons coordinated from the central sulfur atom to the four oxygen atoms. In some text books a structure is shown that suggests octet expansion, however this is simply not necessary.
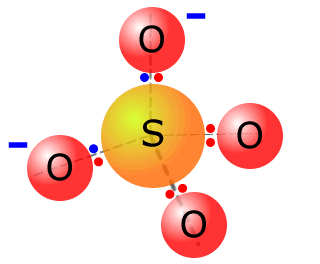 |
Counting up the electrons in the valence shell of the four oxygen atoms and the sulfur we get 5 x 6 = 30. To obtain the structure of the sulfate ion with all outer shells full we need 32 electrons. Thus, the combined atoms have gained two electrons and the ion has a double negative charge. |
|
the sulfate ion SO42-
|
Nitrate ions are formed by reactions of nitric acid with bases and metals.
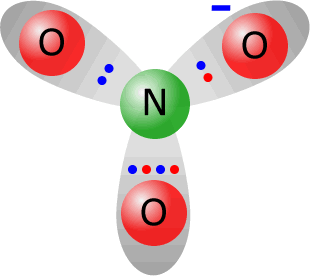 |
Total electrons around the valence shells in the nitrate ion = 24. The sum of valence electrons from the constituent atoms = 5 (from nitrogen) + 3 x 6 (from oxygen) = 23. Thus the ion has gained one electron with respect to its constituent atoms, therefore it must carry a negative charge. Note: All three N-O bonds are the same length and the ion is a totally symmetrical trigonal plane (bond angle 120º. |
|
the nitrate ion NO3-
|
Carbonate ions, CO32-, are the anions formed from carbonic acid (a solution of carbon dioxide in water).
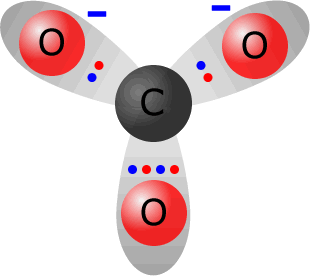 |
Total electrons around the valence shells in the carbonate ion = 24. The sum of valence electrons from the constituent atoms = 4 (from carbon) + 3 x 6 (from oxygen) = 22. Thus the ion has gained two electrons with respect to its constituent atoms, therefore it must carry a double negative charge. Note: All three C-O bonds are the same length and the ion is a totally symmetrical trigonal plane (bond angle 120º. |
|
the carbonate ion CO32-
|
Hydrogencarbonate ions, HCO3-, are formed from carbonate ions by coordinating a hydrogen ion onto one of the oxygen atoms. Sodium hydrogencarbonate, for example, is formed by partial neutralisation of sodium carbonate solution by an acid.
| Na2CO3(aq) + HCl(aq) |
Only group 1 metal hydrogencarbonates are able to exist as solid compounds. The hydrogen carbonates of group 2 metals can be prepared in solution, but decompose when the water evaporates:
| Ca(HCO3)2(aq) |
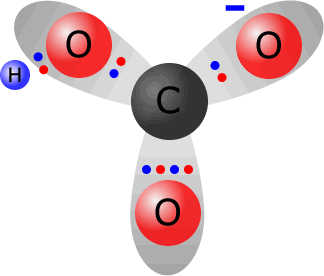 |
Total electrons around the valence shells in the hydrogen carbonate ion = 24. The sum of valence electrons from the constituent atoms = 4 (from carbon) + 3 x 6 (from oxygen) + 1 (from hydrogen) = 23. Thus the ion has gained one electron with respect to its constituent atoms, therefore it must carry a single negative charge. |
|
the hydrogencarbonate ion HCO3-
|
There are many other negative ions encountered in chemistry, but they all conform to the concept of stability due to full outer shells of electrons. The ionic charge can be calculated by examining the difference in electron numbers between the Lewis structure of the ion with full outer shells and the sum of the valence electrons of the constituent atoms.
Polyatomic positive ions (cations)
Stable positive polyatomc ions are not too common, the most common is the ammonium ion. Ammonia is a base with a lone pair of electrons on the nitrogen atom.
.gif) |
.gif) |
|
Ammonia molecule
|
Ammonium ion
|
The lone pair acts as a base and can capture a hydrogen ion to form the ammonium ion according to the equation:
| NH3 + H+ |
The ammonium ion behaves in many ways like a group 1 cation. It forms a whole series of salts, which are all white, soluble and stable at room temperature. However, many of the salts decompose on heating back into ammonia and another molecule.
|
Example: Action of heat on ammonium chloride:
|
Other positive ions found in chemistry include NO+, NO2+, PH4+, CH3+. The only ones stable enough to form compounds are those with complete valence shells around all of the constituent atoms. This is the case for PH4+, but not for CH3+.

