| All matter is originally made of atoms of the 90 (or so) naturally occurring
elements. However, the smallest particles in a pure substance may be: single
atoms, molecules made up of two or more atoms, or ions (atoms, or groups
of atoms, that carry an electrical charge)
The nature of a substance, (the material that we encounter in the everyday world) is a function of (i.e. depends upon) both the types of particles involved and how those particles are bonded (held together) throughout the whole structure. This section deals with the different types of structure, characterised by the bonding existing between the smallest particles |
|
Chemistry is the study of all matter, its physical and chemical properties. However, the majority of matter that we experience in the everyday world is not pure, rather it is a mixture of pure substances that are not chemically bonded together.
Mixtures are inconvenient to study as they usually display the properties of their individual components. It is hard to ascribe a specific property to a particular component without knowing how pure substances behave.
This leads chemists to develop separation techniques designed to provide substances in their pure form for study. Mixtures may be heterogeneous or homogeneous and the type of mixture will determine the procedure employed for separation.
Chemistry is the study of all matter
Simple molecular structures consist of molecules not held to one another by formal bonds. The molecules themselves are constructed by means of covalent (shared electron pair) bonds between non-metal atoms. Molecular structures may normally be identified by their lack of metallic elements in the formula, however their are a few metal compounds that are covalently bonded and which consequently form simple molecular structures.
Examples of these include aluminium chloride, anhydrous iron(III) chloride and beryllium chloride. These anomalies usually involve metal ions with a high charge density, bonded to chlorine.
In the simplest case, the smallest particles in simple molecular structures may consist of single atoms, such as in the noble gases argon and neon etc. As the name suggests, the noble gases are always gases at room temperature.
 |
|
Simple molecular structure
|
As the molecules get larger and more complex the forces between them increase the boiling and melting points. The larger molecular substances are solids at room temperature.
|
Substance
|
Formula
|
Mr
|
Melting point /K
|
Boiling point /K
|
|
Hydrogen
|
H2
|
2
|
14
|
21
|
|
Oxygen
|
O2
|
32
|
55
|
90
|
|
Phosphorus
|
P4
|
124
|
317
|
553
|
|
Iodine
|
I2
|
254
|
387
|
457
|
|
sulfur
|
S8
|
256
|
386
|
718
|
Note: there is a correlation between the relative molecular mass and the melting point although this does not necessarily hold for the boiling point.
We can see that sulfur and iodine with similar Mr values have similar melting points, although the boiling points differ. This is caused by the sulfur molecules changing from S8 crowns to S8 chains after the melting point, which have a greater surface area and hence larger Van der Waal's forces than the simple I2 molecules.
Sulfur consequently requires more energy to vaporise than iodine.
|
|
The whole network (lattice) is a construction of atoms all held together by formal covalent (shared electron pair) bonds. This effectively means that the structure is one giant molecule.
 |
|
Giant molecular structure
|
Examples
|
Substance
|
Formula
|
Mr
|
Melting point /K
|
Boiling point /K
|
|
Graphite
|
C
|
12
|
4003
|
5103
|
|
Silicon dioxide
|
SiO2
|
60
|
1986
|
2503
|
The relative masses in these cases are merely the masses of the simplest formula units. It is clear from the table that no prediction can be made as regards melting and boiling points. Whn these substances melt they do so either with accompanying decomposition (chemical bonds are being broken), or with a change to a simpler molecular substance.
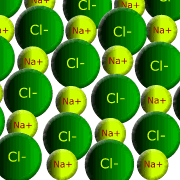
Ionic compounds are formed between metal and non-metal elements. The metal atoms lose electrons forming positively charged ions, while the non-metal atoms gain electrons forming negatively charged ions. These oppositely charged ions are atttracted by strong, non-directional, electrostatic forces to one another and the structure is a giant lattice consisting of repeating units of oppositely charged ions. Water molecules may also be found within the structure to help build the lattice (water of crystallisation)
|
|
Examples
|
Substance
|
Formula
|
Mr
|
Melting point /K
|
Boiling point /K
|
|---|---|---|---|---|
|
sodium chloride
|
NaCl
|
58.5
|
1074
|
1686
|
|
potassium fluoride
|
KF
|
58
|
1119
|
1778
|
The melting and boiling points are governed by the electrostatic forces within the crystal lattice. In turn, the electrostatic force depends on the charge density of the ions; small ions with large charges producing stronger electrostatic forces. Once again, the relative formula mass is really just the mass of the ions in the simplest formula unit (the simplest ratio of ions in the compound)
|
Example: Sodium chloride, NaCl, has a ratio of one sodium ion Na+ per chloride ion Cl- Calcium chloride, CaCl2, has a ratio of one calcium ion Ca2+ to two chloride ions Cl- |
Metallic structures are formed by atoms of metallic elements either alone or mixed with other metallic atoms (alloys). These are giant structures of atoms in which the outer electrons are delocalised throughout the entire structure, effectively leaving the atoms as positively charged ions.
|
|
Examples
|
Substance
|
Formula
|
Ar
|
Melting point /K
|
Boiling point /K
|
|---|---|---|---|---|
|
Sodium
|
Na
|
23
|
371
|
1163
|
|
Potassium
|
K
|
39
|
337
|
1047
|
The melting and boiling points are a function of the forces of attraction between the delocalised electrons and the ions within the metallic lattice. Once again the electrostatic forces are greater when the charge density of the ion is larger. Aluminium, with a three plus charge Al3+ has a high charge density and a much higher melting point than sodium which consists of singly charged and larger ions.
|
Substance
|
Formula
|
ionic radius/pm
|
Melting point /K
|
|---|---|---|---|
|
Sodium
|
Na+
|
116
|
371
|
|
Magnesium
|
Mg3+
|
86
|
923
|
|
Aluminium
|
Al3+
|
68
|
934
|
For the sake of convenience it is easier to deal with giant structures as if the atoms were not bonded. Metals and giant molecular structures can be considered to be a mass of single atoms in which we consider that the simplest ratio between the different types of atoms represents the formula of the substance in question.
|
Example The compound silicon dioxide is a structure of repeating silicon and oxygen atoms arranged in a giant lattice (network). If all the atoms were to be counted it would be found that there are two oxygen atoms for every one silicon atom. We can represent this simplest ratio as SiO2 and call this the formula.
|
This does not mean that there are simple molecules containing only one silicon and two oxygen atoms, it merely gives us a logical way of representing the compound for the sake of convenience and calculations.
There are four fundamental types of structure. These are shown below.
 |
 |
 |
 |
|
simple molecular structure
|
giant molecular structure
|
ionic structure
|
giant metallic structure
|
|
e.g. water
|
e.g. silicon dioxide
|
e.g. sodium chloride
|
e.g. sodium
|
The smallest particle of each type of structure is either an atom, a molecule or an ion. Giant structures require us to be a little more flexible in our thinking as they are effectively one giant molecule.
We define the simplest formula unit to be the simplest ratio of atoms within the giant structure. Similarly, ionic structures must be described according to a simplest formula unit consisting of the simplest ionic ratio.