|
The nature, or properties, of a pure substance is determined by the type of particles that it contains. These may be atoms, molecules or ions. Ionic solids contain a giant lattice (network) of ions, simple covalent solids contain discrete molecules held together by loose forces and giant covalent structures have a lattice (network) of atoms all joined together by strong covalent bonds. Metals may be considered to be a regular arrangement of atoms for the purpose of mass calculations. |

|
The terminology used depends on the type of structure under consideration. There are three ways to describe the relative mass of solids:
- Relative atomic mass (abbreviated Ar)
- Relative molecular mass (abbreviated Mr)
- Relative formula mass (abbreviated Mr)
Where each of these applies is considered below.
1. Relative atomic mass
Relative atomic mass is used to define the relative mass of one atom of a metallic element, or non-metallic element, which may be considered to consist of unique atoms for the purposes of calculations.
|
Inert gases
|
Relative atomic mass |
|---|---|
| Neon - Ne | 20 |
| Argon - Ar | 40 |
|
Giant covalent elements
|
Relative atomic mass |
| Boron - B | 10.8 |
| Silicon - Si | 28 |
| Carbon - C | 12 |
|
Other non-metallic elements
|
Relative atomic mass |
| Phosphorus - P | 31 |
| sulfur - S | 32 |
|
Metals
|
Relative atomic mass |
| Sodium - Na | 23 |
| Magnesium - Mg | 24 |
| Iron - Fe | 56 |
| Uranium - U | 238 |
Note: Although sulfur and phosphorus contain discrete molecules (S8 and P4, respectively), they are usually treated in calculations as monatomic.
2. Relative molecular mass
Relative molecular mass is used to define the relative mass of one molecule of a covalent substance, whether element or compound.
|
Molecular elements
|
Relative molecular mass |
|---|---|
| Hydrogen - H2 | 2 |
| Nitrogen - N2 | 28 |
| Oxygen - O2 | 32 |
| Fluorine - F2 | 38 |
| Chlorine - Cl2 | 71 |
| Bromine - Br2 | 160 |
| Iodine - I2 | 254 |
| simple covalent compounds | Relative molecular mass |
| Ammonia - NH3 | 17 |
| Methane - CH4 | 16 |
| Water - H2O | 18 |
| Hydrogen chloride - HCl | 36.5 |
| Glucose - C6H12O6 | 180 |
| Carbon dioxide- CO2 | 44 |
Note The halogens bromine and iodine are liquid and solid respectively, at room temperature and pressure.
3. Relative formula mass
Relative formula mass is used to define the mass of the simplest ratio of particles in ionic and giant covalent compounds.
|
Ionic compounds
|
Relative formula mass |
|---|---|
| sodium chloride - NaCl | 58.5 |
| magnesium oxide - MgO | 40 |
| potassium nitrate - KNO3 | 101 |
| calcium carbonate - CaCO3 | 100 |
| Giant covalent compounds | Relative formula mass |
| Silicon dioxide - SiO2 | 60 |
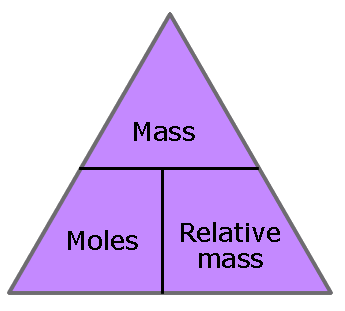
The amount of substance that contains an Avogadro number of particles (6.02 x 1023) is called 1 mole of that substance. Where the word amount is used it always refers to the number of moles.
| metals and other elements Moles = mass / relative atomic mass Simple covalent substances Moles = mass / relative molecular mass Ionic and giant covalent compounds Moles = mass / relative formula mass |
|
|
|
Example 1: Calculate the number of moles in 12 g of magnesium Magnesium has a relative atomic mass = 24 Moles of magnesium in 12 g = 12/24 = 0.5 moles |
|
Example 2: Calculate the amount represented by 15g of silicon dioxide Silicon dioxide, SiO2, is a macromolecular structure with a relative formula mass = 60 Moles of silicon dioxide in 15 g = 15/60 = 0.25 moles |