|
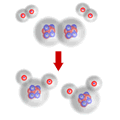
In the previous section it was pointed out that the number of atoms within the particles involved in a chemical reaction cannot change. This idea gives rise to some basic laws of chemical combination that can be used to extract further information about chemical compounds and reactions in general. |
|
The law of conservation of mass
 The
laws of the universe that apply to everyday life are no different for chemistry.
In everyday life only a magician can make things disappear or appear and the
audience knows that in reality nothing has disappeared, it has merely been
an illusion.
The
laws of the universe that apply to everyday life are no different for chemistry.
In everyday life only a magician can make things disappear or appear and the
audience knows that in reality nothing has disappeared, it has merely been
an illusion.
The rabbit that pops out of the magician's top-hat elicits applause and admiration precisely for the reason that it defies common experience and logic.
We know that what we are seeing is impossible, rabbits cannot appear out of thin air!
The girl who disappears from inside the magicians box, likewise is mere trickery, fully grown ladies (or gentlemen for that matter) cannot disappear (with the exception of, perhaps, Lord Lucan!)
In a chemical reaction, the particles that are involved in the beginning of the reaction may break apart and reform, but whatever made them up at the start MUST still be there at the end.
Matter can neither appear nor disappear
This idea may be stated as the "Law of Conservation of Matter", which states that in any process the total mass of the reactants MUST equal the total mass of the products.
Considering the reaction between oxygen and hydrogen:
oxygen + hydrogen ![]() water
water
O2 + 2H2 ![]() 2H2O
2H2O
The mass of water formed MUST equal the mass of oxygen and hydrogen reacting. Notice that this does not necessarily mean the mass of oxygen and hydrogen that are mixed together. Only those molecules that actually react contribute to the mass of water formed.
O2 + 2H2 ![]() 2H2O
2H2O
32g + 4g ![]() 36g
36g
So the mass of water formed is exactly equal to the mass of oxygen and hydrogen reacting.
Reactions evolving gases
When a gas is produced in the course of a chemical reaction it usually escapes and leaves the reaction mixture. The experimentally determined mass of the reactants seems to decrease, but this mass loss is due the mass of the gas that has left.
| Reaction | gas released | |
| Sulfuric acid + magnesium |
hydrogen | |
| Calcium hydroxide + ammonium chloride |
ammonia | |
| Hydrogen peroxide |
oxygen | |
| Manganese(IV) oxide + hydrochloric acid |
chlorine | |
| Hydrochloric acid + sodium carbonate |
carbon dioxide | |
| Copper(II) nitrate |
nitrogen dioxide and oxygen |
Gas evolution allows us to calculate reaction stoichiometry, or formulae, by mass loss or gas volume produced.
|
Example: When an oxide of copper was heated in a stream of hydrogen the following results were obtained.
It seems that mass has been lost from the reaction and as we know that mass cannot be created or destroyed this means that a gas has left the original material. The oxygen has been removed and its mass can be calculated by subtraction. Mass of oxygen leaving = Mass of copper oxide before reaction - Mass of copper remaining after reaction
From these results we can now work out the mole ratio of atoms in the original compound
Therefore the formula of the copper oxide = CuO |
Care must be taken to ensure that all of the gas has really left, as many gases are soluble in water and will be dissolved to a certain extent in any aqueous reaction mixture. This is particularly true for gases such as ammonia or sulfur dioxide, both of which are very soluble in water.
Reactions absorbing gases
When certain substances are heated in air they gain mass, the experimentally determined mass of the reactants increases. This is clearly impossible unless some substance has been added from the suroundings.
Oxygen from the air may react with the substance being heated causing it to gain in mass. The new substance consists of both the original particles and added oxygen particles (occasionally nitrogen or carbon dioxide may also be implicated in mass gain, although this is rather less common)
|
Example: When copper metal is heated in air it turns black and the product has a greater mass than the original copper. Measurement of the mass increase allows us to calculate the formula of the copper oxide produced: Experimental data
Data processing Mass increase due to absorbed oxygen = 1.6g Moles of copper = 6.4/64 = 0.1 moles Moles of oxygen absorbed = 1.6/16 = 0.1 moles Mole ratio of copper to oxygen = 1:1 Therefore formula of copper oxide = CuO |

