|
The equation of state in the previous section refers only to a fixed amount of gas. Avogadro had previously shown that the volume of a gas is proportional to the number of moles. Combining these two ideas gives the ideal gas equation. |
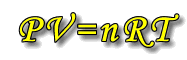
|
Ideal gas equation
The equation of state refers to a fixed mass of gas. From Avogadro's law we know that the same volume of all gases contain the same number of moles and from this, it follows that the volume is proportional to the number of moles.
Volume ∝ number of moles (n)
These two equations can be combined to obtain an expression involving all the quantities:
After rearrangement, for 'n' moles of gas the proportionality constant is called the Universal Gas Constant and is given the symbol 'R'
This gives the ideal gas equation:
Ideal Gas Equation: PV = nRT
where:
- P = pressure in Pa
- V = volume in m3
- n = number of moles of gas
- R = Universal Gas constant = 8.314 JK-1mol-1
- T = the absolute temperature in Kelvin
It is often more convenient to express the pressure in kPa and the volume in litres (dm3). This leaves the value of R the same (see below).
|
Example: Calculate the number of moles of gas present in 2.6 dm3 at a pressure of 1.01 x 105 Pa and 300 K. PV = nRT 2.6 dm3 = 0.0026 m3 0.0026 x 1.01 x 105 = n x 8.314 x 300 n = 0.0026 x 1.01 x 105 / 8.314 x 300 n = 0.105 moles |
There are several units used for gas volume, gas pressure and temperature. It is important to be consistent with the use of units when carrying out gas law calculations. The Syllabus states that SI units will be used wherever possible.
Universal gas constant - R
Although called "Universal", its value depends on the units used for P, V and T.
With the SI units of metres, kilograms, Kelvin and Joules, using P, V and T values at STP gives:
| PV=nRT | |
| therefore: | R=PV/nT |
| for 1 mole of gas at STP (using accepted values of P = 1.00 x 105 Pa, V = 0.02271 m3, T = 273.15 K) | |
| R = | (1.00 x 105) x 0.02271)/273.15 |
| R = | 8.314 J K-1 mol-1 |
In chemistry, the units of volume used are the decimetre cubed (dm3) and pressure in kiloPascals (kPa), so one unit is 100x greater and the other 100x smaller than the SI equivalent. Consequently the differences in the product, PV, both cancel out (multiplying AND dividing by 1000), so that the final value for R is the same as in SI units.
The Universal gas constant, R, calculated using atmospheres Pressure and volume in litres, then:
| PV=nRT | |
| R=PV/nT | |
| at STP: | P = 1 atm, V = 22.7 dm3, T = 273 |
| n = 1 | |
| R = 0.0821 dm3 atm mol-1 K-1 |
There are, of course, several other values of R as there are many ways of measuring both the volume and the pressure of a gas. .
SI units and 'R'
The SI units of P, V and T give rise to the previously used value for the universal gas constant, R = 8.314 J K-1 mol-1.
How does this happen when chemists do not use these SI units?
Remember:
1 litre = 1 dm3 = 1000 cm3
Consequently, if litres are used in the Ideal Gas equation then the pressure units must also be divided by 1000 (as PV = constant). Pressure is measured in Pa or Nm-1, and so the unit of the kPa correct for the difference in volume units.
Atmospheric pressure in Pa = 1.00 x 105 Pa
Atmospheric pressure in kPa = 1.00 x 102 kPa
Provided that you are consistent with the application of units there will be no problem. It is always a good idea when carrying out calculations to look at the value of your answer and ask yourself, "does it seem reasonable?"
The IBO is consistent with the use of litres (dm3) and kPa in gas law questions.

