|
Solutions are one of the commonest ways of performing reactions, as the particles of solute are free to move and collide. It is, however, important for the chemist to know how many particles there are per unit volume of a solution and for this the concept of concentration and molarity are used |
|
Concentration
The concentration of a solution is the quantity of solute that it contains per unit volume.
This may be given in grams per 100cm3 or grams per litre, but it is usually given in terms of molarity as this gives a direct measure of the number of solute particles contained by the solution.
Molarity
The concept of molarity arises from the need to know the amount of solute present in a solution in moles. 1 mole of any substance contains an Avogadro number of particles of that substance = 6.02 x 1023. A 1 molar solution contains 1 mole of solute, dissolved in 1 litre of solution.
Note: the definition is not per 1 litre of solvent, but per 1 litre of solution. This allows us to measure a volume of solution and work out the number of moles, and hence the number of particles, that it contains.
Molarity = number of moles of solute per litre of solution (1 litre = 1000cm3)
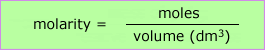
The molarity is denoted by the capital letter M, and given the units mol dm-3
|
Example: Calculate the molarity of a solution containing 0.15 moles of potassium nitrate in 100cm3 of solution. Molarity = moles/litres 100cm3 = 0.1dm3 Molarity = 0.15/0.1 = 1.5 M |
The molarity of a specific ion within an ionic solution may also be considered separately. In a 1M solution of copper sulfate (CuSO4) the copper ions are separate from the sulfate ions. The solution may be said to be both 1 molar in terms of copper 2+ ions and 1 molar in terms of sulfate 2- ions.
A 1 molar (1M) solution of copper nitrate (Cu(NO3)2), however, is 1 molar with respect to copper 2+ ions but 2M with respect to nitrate ions.
|
Example: Calculate the molarity of hydrogen ions in a 0.15 molar solution of sulfuric acid. The formula of sulfuric acid is H2SO4. It dissociates in solution according to the following equation: H2SO4 Hence, if a solution is 1 molar in sulfuric acid, it must be double that in hydrogen ions. Molarity of the solution = 0.15M in sulfuric acid, Therefore the molarity in hydrogen ions = 0.15 x 2 = 0.3 mol dm-3 |
Using:
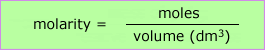
In conjunction with
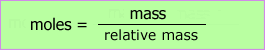
We can calculate the mass needed to prepare solutions or the mass contained in solutions of known concentration.
|
Example: Calculate the mass of iron(II) sulfate in 100 cm3 of 0.1 mol dm-3 solution. The formula of sulfuric acid is FeSO4. It has a relative formula mass = 56 + 32 + 64 = 152 Number of moles in 100cm3 of 0.1 mol dm-3 solution = 0.1 x 0.1 = 0.01 moles Therefore mass of iron /(II) sulfate = moles x relative formula mass = 0.01 x 152 Therefore mass of iron(II) sulfate = 1.52 g |
The first of the above mathematical formulae can be manipulated by rearrangement to obtain any of the three factors, moles, molarity or volume of solution.
Molality
The concept of Molality is NOT required for IB students.