|
Solutions provide an ideal medium for reaction as particles in solution are free to move, allowing frequent collisions. |
|
The dissolution process
Substances dissolve because they form bonds with the solvent molecules. This bond formation (an exothermic process), coupled with the entropy increase on forming a solution, makes the process thermodynamically favourable.
For insoluble substances, the opposite is true; either the bonding within the solid is too strong and requires too much energy to break it, or the bonds formed with the solute molecules are too weak to make the process favourable.
Dissolution, then, is a balance between several factors, making prediction of solubility difficult. Unfortunately, solubility is something that has to be learned, but fortunately, there are a few facts that make the task a little easier.
Reactions occur easily in solution, as the particles are in constant motion and can come into contact with one another. The majority of solutions dealt with in chemistry are ionic. The ions in the structure of an ionic solid are broken apart by interaction with the water molecules. Energy is required to overcome the strong electrostatic forces within the ionic lattice.
 |
 |
 |
|
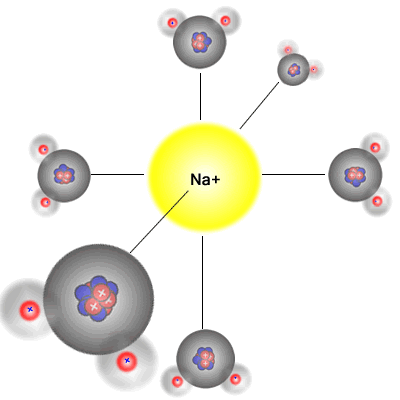
Six water molecules approach
a
positive sodium Na+ ion |
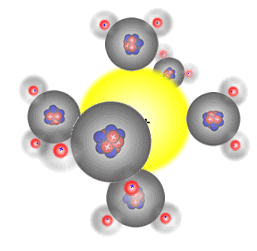
The six water molecules surround
the positive sodium Na+ ion
|
|
This happens because water is a polar solvent with partial positive charges on the hydrogen atoms and a partial negative charge on the oxygen atom. The partially positive hydrogen 'end' of the molecule is attracted to the negative ions (anions), and the partially negative charged oxygen atom in the water molecule is attracted to the positive ions (cations).
The bonds formed between the ions and the solvent water molecules are strong enough to make the process energetically favourable. The energy release on association of water molecules with ions is called the hydration enthalpy of the ions. The smaller the ion the larger its hydration enthalpy. This is dealt with below.
The enthalpy of solution
Water molecules are polar (they have a permanent dipole) and bond to the two types of ion in solution formed when an ionic substance dissolves, surrounding them in an octahedral arrangement. The energy released acts as one of the driving forces behind the dissolution process. However, energy is required to break the crystal lattice of the ionic solid and so any overall energy change (the enthalpy of solution) is the sum of the following three processes.
1. The Lattice enthalpy
Breaking the lattice requires energy, it is an endothermic process. It is represented by the equation:
The stronger the electrostatic forces of attraction between oppositely charged ions, the higher the lattice energy and the more energy required to break the lattice.
2. The Hydration enthalpy of the positive ion (cation)
This is an exothermic (energy releasing) process as the ions are forming bonds with the water molecules. It can be represented by the equation:
3. The Hydration enthalpy of the negative ion (anion)
This is also an exothermic process as the ions are forming bonds (attractions) with the water molecules. It may be represented by the equation:
NOTE: Although written as such above, the hydrated ions are not considered to be complexes in the same way as the transition metal complex ions.
Finally, the process of dissolution involves an increase in entropy of the system as the ions go from their highly ordered crystal lattice to a more disordered arrangement in solution. The Gibb's free energy change of solution determines whether a substance is soluble and, if so, the extent to which it is soluble.
ΔG = Gibb's Free Energy, ΔH= enthalpy of solution, T= Absolute temperature, ΔS= entropy change on dissolution
From the equation you can see that the term -TΔS gets larger as the temperature increases. This means that ΔG gets more negative as the temperature gets higher. The process becomes more favourable (more spontaneous) and solubility usually increases as the temperature rises for this reason.
Ionic reactions - precipitation
If two ions collide that produce an insoluble solid, then a precipitate appears as the new solid is formed. This forms the basis of most simple analytical tests for the presence of ions in solution.
Although it seems as though two new substances have been formed, in fact only the silver chloride can truly claim to have undergone any chemical change. To appreciate this, consider the substances present in the mixture both before and after reaction:
| Species present in solution before reaction | Species present in solution after reaction |
| Na+(aq), Cl-(aq), Ag+(aq), NO3-(aq) | Na+(aq), NO3-(aq), AgCl(s) |
It may be seen that the sodium ions and the nitrate ions do not undergo any change or transformation. They start off in solution and they end up in solution. We call ions such as these spectator ions. The ions simply 'watch' the processes going on around them.
The ionic equation shows only those ions actually involved in reaction forming the white precipitate of silver chloride:
Calculations involving ionic solutions must take into account the stoichiometric relationships between the ions involved in reaction.
|
Example: Calculate the mass of silver chloride precipitated when 25cm3 0.1M silver nitrate reacts with 50cm3 0.1M sodium chloride solution Moles of silver nitrate = 0.025 x 0.1 = 0.0025 moles Moles of sodium chloride = 0.05 x 0.1 = 0.0050 moles From the equation for the reaction:
Cl-(aq) + Ag+(aq)
We can see that moles of Cl- = moles of Ag+ Therefore silver nitrate is the limiting reagent and 0.0025 moles of silver chloride is formed Relative formula mass (silver chloride) = 108 + 35.5 = 143.5 Mass of silver chloride formed = 143.5 x 0.0025 = 0.359g |
Neutralisation reactions
Acids are substances that release hydrogen ions in solution. These hydrogen ions can react in solution with certain other ions, forming water. This removes the hydrogen ions from the solution. We call this type of ionic reaction, neutralisation. Bases contain ions that can react with the hydrogen ions from acids - they neutralise the acids.
Ions that can neutralise acids include:
- hydroxide ions OH-
- oxide ions O2-
- carbonate ions CO32-
- sulfite ions SO32-
Hydroxides
These contain the hydroxide ion usually with a metal ion such as sodium or potassium. Most other metal hydroxides are insoluble and so cannot react with acids from solution. They can however be added as solids to a solution of an acid and neutralise it.
The following reaction shows a solution of sodium hydroxide neutralising hydrochloric acid.
Once again, consideration of the ions present before and after the reaction shows:
| Species present before reaction | Species present after reaction |
| Na+(aq), OH-(aq), H+(aq), Cl-(aq) | Na+(aq), Cl-(aq), H2O(l) |
The sodium ions and the chloride ions are spectator ions, they do not take part in the reaction. The only ions that participate in this reaction are the hydrogen and hydroxide ions:
|
Example: Calculate the volume of 2M hydrochloric acid needed to neutralise 20cm3 1M sodium hydroxide solution Equation for the reaction:
HCl + NaOH
Moles of sodium hydroxide = molarity x volume (litres) = 1 x 0.02 = 0.02 moles From the equation moles sodium hydroxide = moles hydrochloric acid Therefore moles hydrochloric acid = 0.02 moles Volume = moles/Molarity = 0.02/2 = 0.01 dm3 Volume of hydrochloric acid needed = 10cm3 |
More acid - base reactions
Other species that can act as a base by neutralising (removing) the H+ ions in solution are:
|
1 Carbonate ions: CO32- 2H+(aq) + CO32-(aq) 2 Oxide ions: O2- 2H+(aq) + O2-(s) 3 Sulfite (sulfate(IV)) ions: SO32- 2H+(aq) + SO32-(aq) 4 Ammonia (NH3): NH3 + H+(aq) |