|
Ions are also taken to be spherical particles with the radius being the distance between the centre and the outer edge of the ion. |
|
Ionic radius
Ions are particles in the same way as atoms only they carry an electrical charge. This is due to the number of electrons in the outer shells. If a particle has one fewer electrons then it carries a positive charge (there is an excess of protons in the nucleus).
Ions are formed when atoms gain or lose electrons to attain a full outer shell. If an ion loses electrons it also loses an entire shell and becomes much smaller than the parent atom. (This does not apply to transition metal ions).
Positive ions
If loss of electron (s) results in there being one full shell less, then the difference in radius is dramatic.
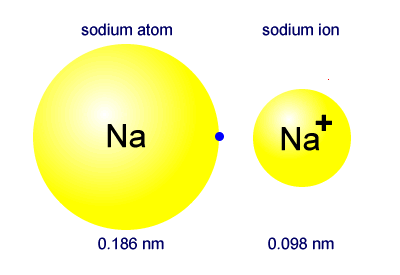
- Sodium atom - electronic configuration 2,8,1 - Atomic radius 0.186 nm
- Sodium ion - electronic configuration 2,8 - Ionic radius 0.098 nm
The sodium ion is approximately half the size of the atom.
Negative ions
Gain of electrons cannot increase the number of shells, but it does increase the inter-electron repulsion and the particle becomes a lot bigger.
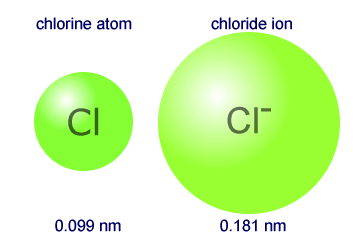
- Chlorine atom - electronic configuration 2,8,7 - Atomic radius 0.099 nm
- Chloride ion - electronic configuration 2,8,8 - Ionic radius 0.181 nm
The chloride ion is approximately double the size of the atom.
Group trends
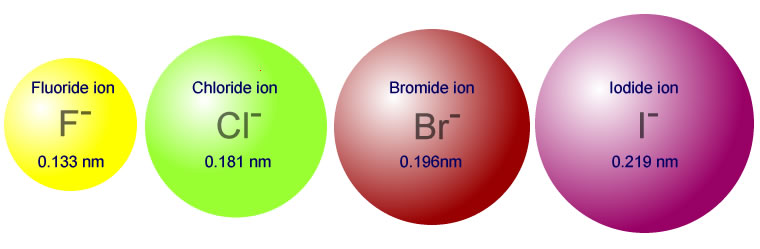
Descending a group the number of electron shells increases and so consequently does the ionic radius. The order for the halide ions, for example is:
Periodic trends
The ionic radius changes radically crossing a period as the elements change from electropositive to electronegative.
Ions formed by the electropositive metals are always positive and involve the atom of the metal losing electrons. The loss of an outer electron shell makes the ion much smaller than the atom. The sodium ion is much smaller than the sodium atom.
The ionic radius changes dramatically crossing a period as the elements change from electropositive to electronegative.
Ions formed by the electronegative non-metals are always negative and involve the atom of the metal gaining electrons to attain a full outer shell (octet).
The gain of outer electrons makes the ion much larger than the atom due to inter electron repulsion. The chloride ion is much larger than the chlorine atom
The consequence is that there is a large jump in ionic radius when the type of ion formed changes from positive to negative at group 15.
Group 14 elements tend not to form ions, so the overall pattern shows a decrease in size from groups 1 to 13 and a large increase from 13 to 15 and a steady decrease from 15 to 17.
The ionic radius increases on decending a group as would be expected with the increasing number of electron shells.
Isoelectronic particles
Isoelectronic means that there are the same number of electrons in the particles.
To compare the radius of isoelectronic particles you need only think about the relative number of protons in each nucleus. More protons equals more force of attraction and a smaller particle. The number of electrons, and hence inter-electronic repulsions and number of shells are always the same.
|
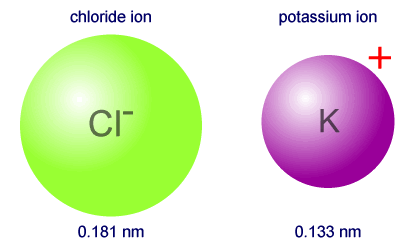
Example: Which of the following isoelectronic particles is the smaller, Cl-, Ar, K+? All of the particles have the same electronic configuration, 2,8,8. However, a potassium ion has 19 protons, argon has 18 protons and a chloride ion has 17 protons. There is a much larger force of attraction from 19 protons, acting on the same number of electrons, making the potassium ion, K+, the smallest of the three particles. |