|
Acids react in a characteristic manner with several types of reagent. |
|
Neutralisation
Acids react wth bases in a neutralisation reaction. Bases are the chemical opposites of acids. This means that they contain ions that can neutralise (react with and cancel out) the H+ ions of the acids.
H+ + OH- ![]() H2O
H2O
The following types of compounds are classified as bases:
|
Base
|
examples
|
formulae
|
| Metal oxides | calcium oxide, magnesium oxide, zinc oxide, iron(II) oxide | CaO, MgO, ZnO, FeO |
| Metal hydroxides | calcium hydroxide, magnesium hydroxide,
sodium hydroxide, potassium hydroxide |
Ca(OH)2, Mg(OH)2, NaOH, KOH |
| Metal carbonates | calcium carbonate, magnesium carbonate,
sodium carbonate, potassium carbonate |
CaCO3, MgCO3, Na2CO3, K2CO3 |
| Metal hydrogen carbonates | sodium hydrogen carbonate, potassium hydrogen carbonate | NaHCO3, KHCO3 |
Neutralisation reactions
Below is a list of neutralisation reactions showing the behaviour of acids with different types of bases. Notice that water is always produced in neutralisation.
|
sulfuric acid
|
+ |
magnesium oxide
|
|
magnesium sulfate
|
+ |
water
|
||
|
H2SO4
|
MgO
|
MgSO4
|
H2O
|
|||||
|
hydrochloric acid
|
+ |
calcium oxide
|
|
calcium chloride
|
+ |
water
|
||
|
2HCl
|
CaO
|
CaCl2
|
H2O
|
|||||
|
sulfuric acid
|
+ |
sodium hydroxide
|
|
sodium sulfate
|
+ |
water
|
||
|
H2SO4
|
2NaOH
|
Na2SO4
|
2H2O
|
|||||
|
hydrochloric acid
|
+ |
calcium hydroxide
|
|
calcium chloride
|
+ |
water
|
||
|
2HCl
|
Ca(OH)2
|
CaCl2
|
2H2O
|
|||||
|
sulfuric acid
|
+ |
zinc carbonate
|
|
zinc sulfate
|
+ |
water
|
+ |
carbon dioxide
|
|
H2SO4
|
ZnCO3
|
ZnSO4
|
H2O
|
CO2
|
||||
|
hydrochloric acid
|
+ |
potassium carbonate
|
|
potassium chloride
|
+ |
water
|
+ |
carbon dioxide
|
|
2HCl
|
K2CO3
|
2KCl
|
H2O
|
CO2
|
||||
|
hydrochloric acid
|
+ |
sodium hydrogen carbonate
|
|
sodium chloride
|
+ |
water
|
+ |
carbon dioxide
|
|
HCl
|
NaHCO3
|
NaCl
|
H2O
|
CO2
|
Note: sulfuric acid always makes salts called sulfates, hydrochloric acid always makes salts called chlorides ( nitric acid makes nitrates, ethanoic acid makes ethanoates - etc.)
Amphoteric behaviour
An amphoteric substance can neutralise both acids and bases. Such substances are exemplified by oxides of 'poor' metals, i.e. metals from the centre of the periodic table, such as aluminium oxide and zinc oxide.
|
Example: The reaction
of aluminium oxide and sodium hydroxide: Al2O3 + 2NaOH Aluminium oxide + sodium hydroxide Example: The reaction of aluminium oxide and sulfuric acid 2Al2O3 + 3H2SO4 aluminium oxide + sulfuric acid |
Reaction with metals
The reaction of metals with acids is often called neutralisation, as the acid gets used up. However, it is nothing of the sort; it is a redox reaction (reduction oxidation). The metal loses its outer electrons and the hydrogen ions from the acid gain electrons to become hydrogen gas. The overall result is a transfer of electrons from the metal to the hydrogen.
M(s) + 2H+(aq) ![]() M2+(aq) + H2(g)
M2+(aq) + H2(g)
This reaction can only take place if the metal is higher in the reactivity series than hydrogen. In other words the reaction does not work with metals less reactive than lead, such as copper and silver.
|
Example: The reaction of magnesium with dilute hydrochloric acid: Mg + 2HCl |
Metals high in the series react very violently with acids and this reaction must not be performed.
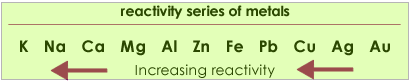
Care must be taken with nitric acid, as it does not behave like a typical acid in its reactions with metals. Nitric acid is a strong oxidising agent and preferentially gets reduced to oxides of nitrogen. It is able to react in this way with most metals, it does not depend on any reactivity series.
|
Example: The reaction of copper with dilute nitric acid: 3Cu + 8HNO3 |
It is easier for the metal to reduce the nitrogen atom than to reduce the hydrogen atom in nitric acid. Some metals, such as iron, become 'passive' when treated with nitric acid. This means that there is an initial reaction which soon ceases as the surface of the metal develops an impervious layer.
Finally, magnesium can cause nitric acid to behave as a normal acid (cf: behaviour of nitric acid with most metals). If the acid is very dilute, hydrogen is evolved.
Indicators
Indicators are substances that change colour in the presence of an acid (or a base). The colour change depends on the type of indicator used. The most common indicators are universal indicator, litmus indicator and phenolphthalein.
The colour changes are:
|
medium
|
Universal indicator
|
Litmus
|
Phenolphthalein
|
|
Acidic
|
|
|
|
|
Neutral
|
|
|
|
|
Basic
|
|
|
|
The degree of acidity or basicity of a solution can be measured by the pH scale. This gives a value proportional to the concentration of H+ ions in the solution