|
This section examines the Brønsted Lowry theory of acids and bases, a theory which extends the traditional Arrhenius definitions of acids. |
|
Brønsted-Lowry
The Arrhenius theory of acids is based on the idea of the behaviour of hydrogen ions in aqueous solution. This was extended by Brønsted and Lowry to include hydrogen ions - protons in any system aqueous or otherwise. They defined acid behaviour as the donation of a proton (hydrogen ion) and base behaviour as acceptance of a proton.
Notice that this does not exclude Arrhenius, it merely extends the idea of acidity to cover non-aquous systems.
Applying Brønsted - Lowry theory to normal acid base reactions, we see that an acid is the species donating the hydrogen ion and the base is the species accepting the hydrogen ion in the aqueous system. Hence Arrhenius' theory of aqueous acidity is encompassed by that of Brønsted and Lowry.
CH3COOH + OH- ![]() CH3COO- + H2O
CH3COO- + H2O
In this example, the ethanoic acid is donating the proton (hydrogen ion) and the hydroxide ion (base) is accepting it. Ethanoic acid is behaving as an acid according to both Arrhenius and Brønsted-Lowry.
Although this is a rather trivial example, it serves to highlight the fact that the Brønsted-Lowry theory is just and extension of Arrhenius theory. Where it is useful is in its application to non-aqueous systems, where Arrhenius' cannot be applied.
|
Example: In the reaction between ammonia gas and hydrogen chloride the hydrogen chloride transfers a hydrogen ion to the ammonia making an ammonium ion. In this reaction the ammonia is behaving as a Brønsted Lowry base by accepting a proton (hydrogen ion). The hydrogen chloride is a Brønsted-Lowry acid for providing (donating) that proton (hydrogen ion). HCl + NH3 |
Any system where hydrogen ions are transferred can be considered according to the Brønsted Lowry definition of acids and bases.
Brønsted-Lowry acids
In order to consider the possibility of a substance behaving as a Brønsted-Lowry acid or base you must consider the possibility of it accepting or donating hydrogen ions.
The species under consideration must have a hydrogen atom that is capable of being detached as a hydrogen ion.
NH4+ can behave as a Brønsted-Lowry acid as it can lose an H+ and become NH3.
Brønsted-Lowry bases
These must be capable of accepting a hydrogen ion into the structure.
In the reaction between sulfuric acid and nitric acid, the sulfuric acid donates a proton to one of the oxygens of the nitric acid. It is the first stage of formation of the nitronium ion NO2+
In this reaction sulfuric acid is behaving as a Brønsted-Lowry acid and the nitric acid is a Brønsted-Lowry base.
H2SO4 + HNO3 ![]() [H2NO3]+ + HSO4-
[H2NO3]+ + HSO4-
[H2NO3]+ ![]() H2O + NO2+
H2O + NO2+
Conjugate acid - base pairs
The idea of conjugate acid - base pairs comes from the idea that all reactions are fundamentally reversible (if not in practice, at least in theory). When a proton is transferred to another species, that product is then capable, in turn, of transferring the proton back - in other words it is itself capable of behaving as an acid.
As this acid was created by accepting the proton in the first place it is called the conjugate (paired) acid of the original base. We say that the two species form an acid - conjugate base pair.
- An acid on the left hand side always has a conjugate base on the right hand side
- A base on the left hand side always has a conjugate acid on the right hand side
- These pairs of species are called conjugate acid - base pairs
|
Example: Finding the conjugate partner in a reaction involves considering the reverse reaction or considering where the proton (hydrogen ion) goes to. In the following example consider the hydrogen ion and remember that the hydrogen ion donator is the acid. CH3COOH + H2SO4 On the left hand side it is the sulfuric acid that donates the proton. It is behaving as an acid. Its conjugate base is the species on the other side of the equation that would accept the proton to go in the reverse direction. On the right hand side it would be the hydrogen sulfate ion (HSO4- ) that accepts the proton and therefore it is the conjugate base of the sulfuric acid. On the left hand side of the equation the ethanoic acid accepts a proton and is therefore the Brønsted-Lowry base. It has a conjugate acid on the right hand side that can release a proton - this is the CH3COOH2+ion. |
Conjugate acid - base pair examples
Look at the following equations as examples of conjugate acid - base pairs. Remember that the conjugate partners are found by considering the reverse reaction.
|
acid
|
base
|
conjugate base
|
conjugate acid
|
|||
|
H2SO4
|
+
|
CH3COOH
|
|
HSO4-
|
+
|
CH3COOH2+
|
|
CH3COOH
|
+
|
NH3
|
|
CH3COO-
|
+
|
NH4+
|
|
H2O
|
+
|
H2O
|
|
H3O+
|
+
|
OH-
|
|
HCl
|
+
|
H2O
|
|
Cl-
|
+
|
H3O+
|
|
proton donor
|
proton acceptor
|
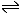 |
proton acceptor
|
proton donor
|
Logically, if the reactions involve the transfer of a proton (hydrogen ion) from the acid to a base the structures of the acid and its conjugate base must differ in structure by that hydrogen ion.
Look at the acid and conjugate base in the above equations, and ou can see that the acid-conjugate base pairs differ by one hydrogen ion, as do the base-conjugate acid pairs.
The Syllabus states that the position of the hydrogen ion should be indicated. The hydrogen ion being transferrred to the base is accepted by the base. It must be accepted in a location where there is a lone pair of electrons to hold it.
In the first equation above, it is the lone pair on the oxygen atom of the ethanoic acid that accepts the proton. It is therefore correct to write the formula of the conjugate acid of the ethanoic acid (base) as CH3COOH2+, as this indicates that the proton is accepted by the oxygen (or one of them) of the carboxyl group. It would be incorrect to write the formula as CH4COOH+ as this would suggest that the hydrogen has been accepted by the wrong part of the structure.