|
Prior to the discovery by Freidrich Wöhler in 1828 that urea could be made by evaporation of ammonium thiocyanate solution, it was thought that organic chemicals could only be produced by the hand of a God. Nowadays our definitions of organic chemistry are not quite so stringent... |
Carbon compounds
Carbon atoms possesses some remarkable properties.
- They can form strong, stable covalent bonds to other carbon atoms and many different types of atom.
- They can build up chains of carbon atoms to form a carbon 'skeleton'. (catenation - literally 'chain making')
- They can form multiple bonds, both with other carbon atoms or chains, and with other elements.
These three factors allow carbon to produce, literally, millions of different compounds, many of which are found in living systems. The definition of organic compound is now taken to mean a compound of carbon that is not a simple mineral compound. Again, this is pretty vague, but means that carbonates, hydrogen carbonates and oxides are excluded from the definition.
Carbon is often bonded to hydrogen and oxygen in its compounds. Those compounds that contain carbon and hydrogen only are called hydrocarbons.
Bonding in carbon compounds
Carbon has four electrons in its highest energy level (outer shell). It must share four more electrons to attain a full outer shell. There are four ways that carbon atoms can do this.
- By forming four covalent bonds to four other atoms.
- By bonding covalently to two other atoms using one shared pair of electrons and a third atom using two shared pairs of electrons (a double bond)
- By bonding covalently to two other atoms by means of two shared pairs of elctrons (two double bonds - this is unusual)
- By bonding covalently to two other atoms, one using a single bond and to the other by means of a triple bond (three shared pairs)
Carbon single bonds are of the type sigma, caused by direct orbital overlap along a linear axis.
Carbon double bonds consist of one sigma bond and one 'pi' bond, caused by lateral (sideways) overlap of two parallel orbitals. Notice that the overlap happens above and below the sigma bond. These two overlaps constitute only one pi bond.
Triple bonds formed by carbon atoms comprise one 'sigma' and two 'pi' bonds. The 'pi' bonds are at right angles to one another. In a triple bond with two 'pi' bonds there are four regions of overlap corresponding to the two pi bonds and one region of overlap along the axis joining the two atoms corresponding to the sigma bond.
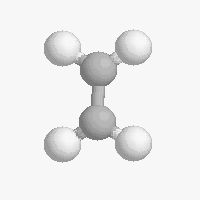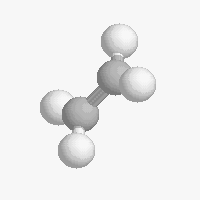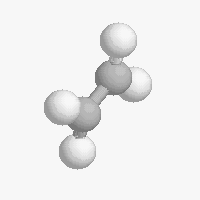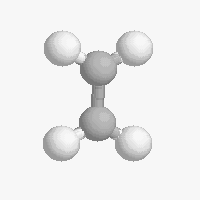
Shapes of organic molecules
The shape of a molecule depends on the geometry of the bonding electrons that join all of the atoms together. Carbon atoms may adopt one of three geometries depending on the number of atoms to which they are bonded.
| The geometry of a four bonded carbon is tetrahedral with bond angles of 109.5º. | The geometry of carbon bonded to three other atoms is trigonal planar (bond angle 120º) | The geometry of carbon bonded to two other atoms is linear (bond angle 180º) |
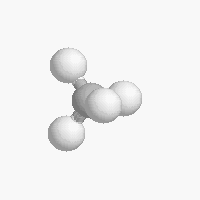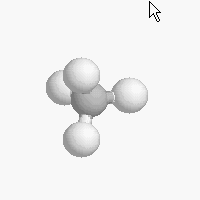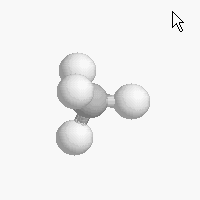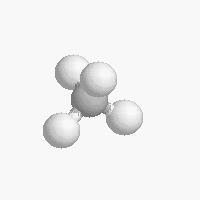 |
 |
 |
Functional groups
The average bond strength of a carbon - carbon single bond is 346 kJ mol-1 and a carbon = carbon double bond has a bond strength of 602 kJ mol-1.
To break carbon-carbon bonds requires large amounts of energy, meaning that the carbon skeleton is particularly stable. Organic compounds react in many different ways, but the carbon skeleton is rarely broken. For this reason, the atoms that are attached to the carbon skeleton are very important as they often confer reactivity to the molecule. Such atom or groups are called 'functional groups' - they give the molecule functionality.
Common functional groups
| Atom or grouping | name |
| -Cl | chlorine atom |
| -F, -Cl, -Br, -I | halide 'group' |
| -OH | alcohol group |
| -CHO | aldehyde group |
| -COOH | carboxylic acid group |
| -NH2 | amine group |
| -CONH2 | amide group |
| -CN | nitrile group |
Groups or atoms may also be used to join two carbon chains together. Such groups are sometimes called 'linkages'
Linkage groups
| Linking atom or group | name |
| O | ether link |
| -COO- | ester link |
| -NHCO- | amide link |
| -O-O- | peroxide link |

