|
A system of abbreviating the atoms to show the fundamental sub-atomic particles within. |
The atomic mass number is represented by the symbol (letter) 'A'. This is not to be confused with the relative atomic mass Ar.
The mass number gives the integral number of nucleons, protons and neutrons found in the nucleus of an atom.
The relative mass is a value that is not necessarily integral that compares a mass to the mass of a carbon isotope, assigned a value of exactly 12.0000 units.
This is represented by the symbol (letter) 'Z'. It shows us the number of protons in an atom (and the number of electrons in a neutral atom.)
|
Example: How many protons and electrons does an atom of iron contain? The atomic number of iron is 26 therefore it contains 26 protons The number of electrons = number of protons, therefore there are 26 electrons |
Any isotope of any element can be defined by using the A value, the Z value and the element symbol.
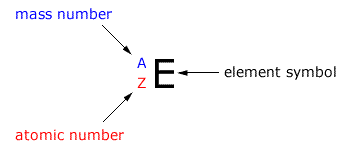
Using the values of A and Z it is possible to calculate the number of sub-atomic particles within any specific isotope of an element.
|
Example: Determine
the number and type of sub-atomic particles in the following atom:
The atomic number is 1 therefore there is 1 proton and 1 electron The mass number is 3 therefore there are (3-1) = 2 neutrons |
The system can be extended to cover ions simply by adding the charge onto the element symbol. It is important to remember that a positive ion has LOST electrons.
|
Example: Determine
the number of electrons in the following ion: The atomic number is 12 therefore in a neutral atom there would be 12 protons and 12 electrons. However, the charge is 2+ therefore the atom has LOST 2 electrons The remaining electrons then = 10 electrons |
Answer
|
The number of protons is represented by the atomic number on the AZE system - in this case the red number 5. Therefore this boron atom has 5 protons |
Q113-02 How many electrons are present in the atom
Answer
|
The number of electrons in a neutral atom (as opposed to an ion) is given by the atomic number on the AZE system - in this case the red number 13. Therefore this aluminium atom has 13 electrons |
Q113-03 How many neutrons are present in the atom
Answer
|
Argon has a mass number of 40 as shown by the blue number. The mass number is given as the sum of the protons and the neutrons. The protons are obtained from the red number 20, the atomic number. Therefore each argon atom contains 40-20 = 20 neutrons |
Q113-04 The atom
Answer
|
This technetium atom has an atomic number of 43 and a mass number of 99. It contains 99-43 = 56 neutrons |
Q113-05 An ion with 5 protons, 6 neutrons, and a charge of 3+ has an atomic number of:
- 5
- 6
- 8
- 11
|
Here the 3+ ion is merely a distraction. The atomic number is obtained from the number of protons, in this case 5. Therefore correct response = A |
Q113-06 One 40Ca2+ ion contains:
- 2 protons
- 18 electrons
- 21 neutrons
- 2 electrons
|
In a neutral atom the electrons = the protons = the atomic number, in this case 20. The calcium ion has lost two electrons, therefore number of electrons = 18 Correct response = B |
Q113-07 The symbol for a particular magnesium ion is 24Mg2+. The number of electrons contained is one of these ions is:
- 2
- 10
- 12
- 22
|
In the magnesium ion, two electrons have been lost (leaving an overall double positive charge). In a neutral atom, the electrons = the protons = the atomic number, in this case 12. Therefore the total number of electrons = 12 - 2 = 10 Correct response = B |
Q113-08 How many neutrons are present in the ion
Answer
|
Ionic charge affects only the electrons in a particle. This strontium particle has a mass number of 90 and an atomic number (proton number) of 37. Therefore number of neutrons = 90 - 37 = 53 neutrons |
Q113-09 How many electrons are present in the ion
|
Cobalt has an atomic number of 27. In a neutral atom it has 27 electrons, however, in the Co3+ ion it has LOST three electrons. Therefore number of electrons left = 24 electrons. |
Q113-10 How many neutrons are present in the atom
Answer
|
Potassium has a mass number of 39 as shown by the blue number. The mass number is equal to the sum of the protons and the neutrons. The protons are obtained from the red number 19, the atomic number. Therefore each potassium atom contains 39-19 = 20 neutrons |

