|
Mass spectrometry is not just devoted to finding the relative molecular mass of elements, but is also used as a sophisticated component of many analytical systems in association with chromatography and other techniques. |
|
Relative molecular mass
The mass spectrometer is an instrument used for three main purposes:
- 1 Measuring the exact relative masses of elements (section 1.22).
- 2 Measuring exact releative molecular formula mass
- 3 Measuring the masses of the breakdown products from molecules when they are smashed to pieces by high energy electrons. This is also called the fragmentation pattern and may be useful in elucidation of the structure of a molecule.
Accurate mass measurement
The ions can be focussed onto the detector electronically. This allows determination of the m/e of the ion, to an accuracy of 8 decimal places. The mass of the electron that has been lost can be taken into account, giving masses for the atoms that are very accurate.
Molecular formulae determination
The masses of the atoms can be found to such great accuracy that the mass of each molecule becomes unique, according to the number and type of each atom present.
|
Example: The relative molecular mass of carbon monoxide = C + O = 28 (to two significant figures places) The relative molecular mass of nitrogen = N + N = 28 (to two significant figures places) But, using high resolution mass spectrometry
It should be appreciated that the following peaks are easily differentiated:
|
Structural information
Spectra of molecules are rather more complex due to the breakup (fragmentation) of the molecule in the electron beam.
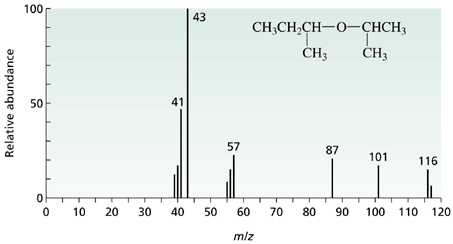
Here we can see that there is a fragmentation pattern caused by the molecule breaking apart in the electron bombardment.
The molecule is shown on the spectrum and the most important peak is the one at m/e = 116 which gives the relative molecular mass of the molecule. This peak is said to be due to the "molecular ion" and is caused by the molecule itself losing only one electron before going to the detector.
The m/e value of the molecular ion can be measured to such a degree of accuracy (many decimal places) that it can be used to determine the exact number of each type of atom within the molecule.
A full treatment of the fragmentation pattern is possible to give information regarding how the molecule is bonded together, but not required for this section.

