|
Electromagnetic energy travels at the speed of light in the form of waves. These waves are oscillations in the electrical field and the corresponding magnetic field which is at right angles to it. Electromagnetic waves travel at the speed of light. The wave may be characterised by its wavelength and frequency. Syllabus referenceStructure 1.3.1 - Emission spectra are produced by atoms emitting photons when electrons in excited states return to lower energy levels.
Guidance
Tools and links |
Waves are defined by velocity, wavelength, frequency and amplitude. This is shown in the following diagram:
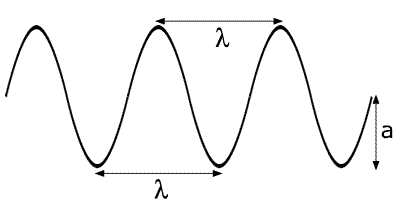
 The wavelength is represented by the Greek letter
lambda - l
The wavelength is represented by the Greek letter
lambda - l The frequency is the number of wavelengths that
pass per second - f (sometimes the Greek letter 'nu', n).
This is measured in Hertz (Hz) or cycles per second.
The frequency is the number of wavelengths that
pass per second - f (sometimes the Greek letter 'nu', n).
This is measured in Hertz (Hz) or cycles per second. The velocity of the wave in the case of electromagnetic
radiation is the speed of light 'c' (3 x 108 ms-1 [data
booklet])
The velocity of the wave in the case of electromagnetic
radiation is the speed of light 'c' (3 x 108 ms-1 [data
booklet]) The amplitude of the wave is represented by the
letter 'a'. It is a measure of the intensity and is relatively unimportant.
The amplitude of the wave is represented by the
letter 'a'. It is a measure of the intensity and is relatively unimportant.
These wave properties are related by the equation:
![]()
The energy carried by an electromagnetic wave depends on the wavelength - they are inversely proportional, i.e. the shorter the wavelength the higher the energy.
However, as the speed of the electromagnetic radiation is always constant, the energy is also directly proportional to the frequency of the wave. Higher frequency = greater energy. This can be shown by the following equation:
![]()
Where 'h' is called Planck's constant (6.63 x 10-34 Js [data booklet])
|
Example: Calculate the frequency of radiation emitted by an IR source with a wavelength of 1500 nm. The speed of light, c = lf Hence: f = c/l = 3 x 108/1500 x 10-9 Hence frequency of radiation = 2 x 1014 Hz |
The wavelength of electromagnetic radiation varies according to its energy. The table below shows the terms used for different regions of electromagnetic radiative energy and their approximate wavelength and frequency ranges.
| Frequency | Wavelength | Type of electromagnetic radiation | Energy |
| 1024 | 3 x 10-16 | Gamma rays |
high to low |
| 1021 | 3 x 10-13 | far X-rays | |
| 1018 | 3 x 10-10 | X-rays | |
| 1015 | < 3 x 10-7 | Ultraviolet | |
| 1015 | 3.6 - 7.6 x 10-7 | Visible | |
| 1015 | > 3 x 10-7 | Infrared | |
| 1012 | 3 x 10-4 | Microwaves | |
| 109 | 3 x 10-1 | UHF television | |
| 106 | 3 x 102 | Radio waves |
Note that the visible region of the spectrum, i.e. the region that human eyes are able to detect, is incredibly small when compared to the whole range.
Effectively, there is no upper or lower limit to the wavelength and the energy.
