|
Hybridisation is a word that strikes fear into the very heart of students. However, in reality the concept is relatively straightforward. In this section we explore the concept of hybridisation and learn to recognise and use it. |
|
Background
Science goes forward by means of a process of observation, experimentation and theorisation. The theories and explanations for observations are considered valid providing there is no evidence to the contrary.
Through the work of Schroedinger and others in the middle of the 20th century, scientists began to put shapes to the orbitals that hold the electrons around atoms. However the shape and orientation of the atomic orbitals did not explain the shapes of molecules. It was clear that some theory was needed to explain how atomic orbitals could somehow give rise to the shapes of the molecules formed from them.
There are various approaches to the problem, one of which is the concept of hybridisation.
Linear combination of atomic orbitals
Covalent bonds involve shared pairs of electrons. These shared pairs using consist of one electron from one atom and the second electron being provided by the other atom. Clearly these two electrons are going to have to be in the same region of space. As they are held in atomic orbitals these orbitals themselves must overlap.
Sigma bonds - direct orbital overlap
When the process of orbital overlapping involves a direct overlap along an axis the bond is referred to as a sigma bond. Sigma bonds are always single bonds. One electron is usually provided by each of the overlapping orbitals, although in certain cases both of the electrons may be donated by the same atom. The second case is called dative covalent bonding.
Pi bonds - lateral orbital overlap
Pi bonds can only form AFTER sigma bonds have already formed. A pi-bond cannot exist on its own. Sigma bond formatin brings suitable orbitals into sideways (lateral) contact on the adjacent atoms. This causes orbital overlap both above and below the axis of the sigma bond. This lateral overlap of two parallel orbitals contributes to the force of attraction between the two atoms - it is called a pi-bond.
|
|
Hybridisation
This is the idea that atoms involved in bonding rearrange their orbitals shapes and energies in order to produce orbitals that can overlap successfully with suitable orbitals on adjacent atoms and be used in bonding.
Carbon, for example, forms four bonds with hydrogen to make the molecule CH4. Studies show that the methane molecule is symmetrical and the hydrogen atoms are arranged in a tetrahedral fashion around the central carbon atom.
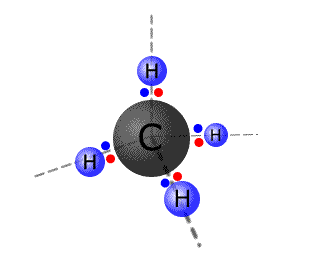 However,
the atomic orbitals in the outer shell of carbon have two electrons in a 2s
orbital and 2 electrons in two 2p orbitals. There are three problems here:
However,
the atomic orbitals in the outer shell of carbon have two electrons in a 2s
orbital and 2 electrons in two 2p orbitals. There are three problems here:
- The 2s orbital is spherical and can't overlap with adjacent atoms.
- The 2s orbital is full. It can't share an electron from another overlapping orbital.
- There are only two singly occupied orbitals available for overlap and shaing electrons suggesting that carbon should only form two bonds and that these bonds would be at 90º to one another.
Carbon atoms overcome these problems by reforming the orbitals to give four degenerate orbitals, each of which is singly occupied. This is called hybridisation.
It must be stressed that hybridisation is simply an model to explain the difference between the shapes and orientation of the orbitals found in atoms and molecules. It gives us a process that explains how one situation can change into another.
Hybridisation in double and triple bonds
Double bonds
Hybridisation can also explain the formation and shape of molecules that contain double and triple bonds.
Using carbon as the example, in the case of a double bond the central atom needs to only bond to three other atoms. To do this it needs three degenerate orbitals which it obtains by hybridisation of the three valence orbitals that have the lowest energy, i.e. the 2s and two of the 2p orbitals.
Triple bonds
Atoms that are triple bonded can only bond to two other atoms. This means that two degenerate orbitals are needed. These are provided by hybridisation of the two orbitals from the valence shell that have the lowest energy, i.e. the 2s and onle of the 2p orbitals.
This hybridisation scheme produces two 'sp' orbitals at 180º to one another (linear) that can be used for direct orbital overlap (sigma bonding) and leaves two unchanged 'p' orbitals that can be used for lateral overlap with suitable orbitals on adjacent atoms (pi bonding).
It must be stressed that hybridisation occurs in all molecules, not just carbon-containing molecules. It is a simple matter to spot sp3, sp2 and sp hybridisation by the number and type of bonds on an atom.
Carbon, for example, forms four bonds. If all four bonds are single then the hybridisation is sp3. If there is one double bond then it is using sp2 hybridisation and if there is a triple bond then the hybridisation is sp.
Nitrogen forms only three bonds with other atoms and in nitrogen containing
compounds one double bond is indicative of sp2 hybridisation, just
like in carbon. In the ammonia molecule the four electron pairs are arranged
in a tetrahedral orientation using sp3 hybridisation. 
In the hydrogen cyanide molecule the nitrogen is attached to the carbon atom by a triple bond. Both atoms are sp hybridised.
In summary:
- If an atom has only single bonds then it is using sp3 hybridisation.
- if an atom is attached with one double bond then it is sp2 hybridised
- If an atom is attached by a triple bond then it is sp hybridised.

