|
The force of gravity acts on all matter and given a chance would pull all particles to the earth's surface. However, the particles of matter seem to defy this gravitational force and the only explanation is that they too have forces of attraction holding them together. |
|
Matter tends to stick together. We know this because solids and liquids exist!
This tells us that there are forces of attraction between particles of matter, the so-called inter-particular forces. The types of particles vary from one compound to another, but there is only one fundamental force of attraction, which is electrostatic in nature, the force of attraction betweeen partial, or total, positive and negative charges.
Positive charges attract negative charges and vice versa.
Intermolecular forces in covalent molecules
When the particles that we are dealing with are molecules, the forces of attraction between the molecules, the interparticular forces, are called intermolecular forces. Covalent molecules are electronically neutral, so where do the positive and negative charges that give rise to electrostatic attraction come from?
The way forces are created is determined by the polarity of the compound. There are only two basic mechanisms.
However, permanent dipole - dipole interactions may be subdivided into:
- Permanent dipole-dipole interactions
- Hydrogen bonding (a special case of permanent dipole-dipole)
Induced dipole - dipole forces of attraction (also known as London dispersion forces) exist between ALL particles. It is thought that they are due to vibration of the nucleus within the negative charge cloud, creating polarity of temporary positive and negative charge within molecules.
The vibrations set up sympathetic vibrations in neighbouring molecules that brings opposite partial charges into close proximity. It is this that causes the attraction between molecules.
This temporary state may cause attraction between two molecules, pulling them together. The magnitude of dispersion force depends on the relative molecular mass, high mass produces a larger force.
That dispersion forces can be strong is often ignored when dealing with intermolecular forces. However, as molecules get larger it becomes the dominant force. The important thing is not to cause confusion. Out of the possible intermolecular forces, all other things being equal, dispersion forces are the weakest, but this doesn't mean that they are necessarily weak.
In sulfur and iodine, for example, the force is strong enough to cause them to be solids at room temperature. The relative molecular mass of sulfur is 256 and the relative molecular mass of iodine is 254.
 |
 |
| Sulfur Mr 256 m.p. = 113ºC | Iodine Mr 254 m.p. = 114ºC |
Notice the similarity between the relative masses and the melting points of sulfur and iodine. The slight difference can be attributed to the different molecular shapes giving rise to slightly different volume to surface area ratios (see below) in the two types of molecule.
|
Example: Explain, by reference to the intermolecular forces, why sulfur has a higher melting point than phosphorus. Both sulfur and phosphorus are non-polar covalent solids. The only force of attraction that exists between molecules of both kinds is dispersion force. The strength of this force is dependent primarily on the relative molecular mass. Sulfur exists as S8 molecules with a relative mass of 256. Phosphorus exists as P4 molecules with a relative mass of 124. Sulfur has a much higher relative mass than phosphorus, stronger dispersion forces and, because of this, a higher melting point. |
The homologous series that characterise organic chemistry allow us to see clearly the relationship between relative mass and dispersion forces. A graph plotted of mass against boiling point gives a (very nearly) straight line.
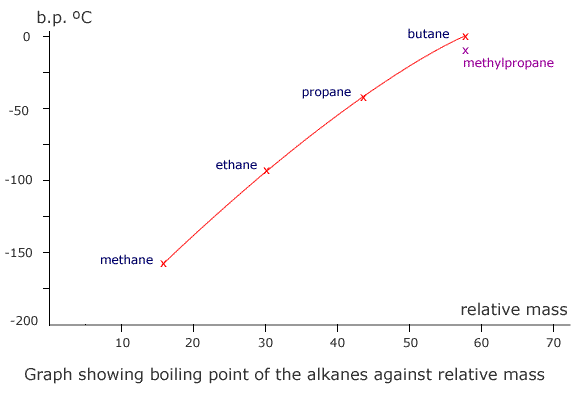
Notice that methylpropane, CH3CH(CH3)CH3, has a lower boiling point than its straight chain isomer, propane, CH3CH2CH2CH3. This is due to the 'fine-tuning' of dispersion forces by the surface area to volume ratio difference between the two molecules.
 |
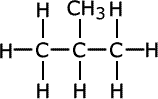 |
| butane | methylpropane |
Geometrically speaking, the shape with the lowest surface are to volume ratio is the most volatile as dispersion forces act at the surface of molecules, so the greater the surface area, the stronger the force (once again, all other things being equal).
A branched chain is a closer approximation to a sphere than a straight chain. Hence, straight chains have a higher boiling point than branched chain molecules, they have a larger surface area at which attractions occur.

