Standard level
The force of gravity acts on all matter and given a chance would pull all particles to the earth's surface. However, the particles of matter seem to defy this gravitational force and the only explanation is that they too have forces of attraction holding them together.
Syllabus ref: S2.2.8Structure 2.2.8 - The nature of the force that exists between molecules is determined by the size and polarity of the molecules.
- Intermolecular forces include London (dispersion), dipole-induced dipole, dipole–dipole and hydrogen bonding.
- Deduce the types of intermolecular force present from the structural features of covalent molecules.
Guidance
- The term “van der Waals forces” should be used as an inclusive term to include dipole–dipole, dipole induced dipole, and London (dispersion) forces.
- Hydrogen bonds occur when hydrogen, being covalently bonded to an electronegative atom, has an attractive interaction on a neighbouring electronegative atom.
Tools and links
- Structure 1.5 - To what extent can intermolecular forces explain the deviation of real gases from ideal behaviour?
- Nature of science, Structure 1.1, Structure 2.1, Structure 2.3 - How do the terms “bonds” and “forces” compare?
- Nature of science - How can advances in technology lead to changes in scientific definitions, e.g. the updated International Union of Pure and Applied Chemistry (IUPAC) definition of the hydrogen bond?
- Forces between particles
- Intermolecular forces between covalent molecules
- Dispersion forces
- Trends in organic chemistry
- Permanent dipole - dipole interactions
- Comparison with dispersion forces
- Hydrogen bonding
- The group 15, 16 and 17 hydrides
- Comparing molecules
- Dimerisation
- Solubility
- Worked examples
Forces between particles
Matter tends to stick together. We know this because solids and liquids exist!
This tells us that there are forces of attraction between particles of matter, the so-called inter-particular forces. The types of particles vary from one compound to another, but there is only one fundamental force of attraction, which is electrostatic in nature, the force of attraction betweeen partial, or total, positive and negative charges.
Positive charges attract negative charges and vice versa.
Intermolecular forces between covalent molecules
When the particles that we are dealing with are molecules, the forces of attraction between the molecules, the interparticular forces, are called intermolecular forces. Covalent molecules are electronically neutral, so where do the positive and negative charges that give rise to electrostatic attraction come from?
The way forces are created is determined by the polarity of the compound. There are only two basic mechanisms.
- Temporary dipole - dipole interactions (London dispersion forces)
- Permanent dipole dipole interactions
However, permanent dipole - dipole interactions may be subdivided into:
- Permanent dipole-dipole interactions
- Hydrogen bonding (a special case of permanent dipole-dipole)
Dispersion forces
Induced dipole - dipole forces of attraction (also known as London dispersion forces) exist between ALL particles. It is thought that they are due to vibration of the nucleus within the negative charge cloud, creating polarity of temporary positive and negative charge within molecules.
The vibrations set up sympathetic vibrations in neighbouring molecules that brings opposite partial charges into close proximity. It is this that causes the attraction between molecules.

This temporary state may cause attraction between two molecules, pulling them together. The magnitude of dispersion force depends on the relative molecular mass, high mass produces a larger force.
That dispersion forces can be strong is often ignored when dealing with intermolecular forces. However, as molecules get larger it becomes the dominant force. The important thing is not to cause confusion. Out of the possible intermolecular forces, all other things being equal, dispersion forces are the weakest, but this doesn't mean that they are necessarily weak.
In sulfur and iodine, for example, the force is strong enough to cause them to be solids at room temperature. The relative molecular mass of sulfur is 256 and the relative molecular mass of iodine is 254.
 |
 |
| Sulfur Mr 256 m.p. = 113ºC | Iodine Mr 254 m.p. = 114ºC |
Notice the similarity between the relative masses and the melting points of sulfur and iodine. The slight difference can be attributed to the different molecular shapes giving rise to slightly different volume to surface area ratios (see below) in the two types of molecule.
|
Example: Explain, by reference to the intermolecular forces, why sulfur has a higher melting point than phosphorus. Both sulfur and phosphorus are non-polar covalent solids. The only force of attraction that exists between molecules of both kinds is dispersion force. The strength of this force is dependent primarily on the relative molecular mass. Sulfur exists as S8 molecules with a relative mass of 256. Phosphorus exists as P4 molecules with a relative mass of 124. Sulfur has a much higher relative mass than phosphorus, stronger dispersion forces and, because of this, a higher melting point. |
Trends in organic chemistry
The homologous series that characterise organic chemistry allow us to see clearly the relationship between relative mass and dispersion forces. A graph plotted of mass against boiling point gives a (very nearly) straight line.
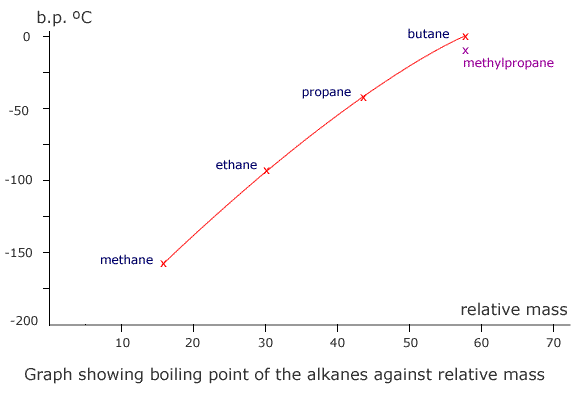
Boiling points of some organic molecules
Notice that methylpropane, CH3CH(CH3)CH3, has a lower boiling point than its straight chain isomer, propane, CH3CH2CH2CH3. This is due to the 'fine-tuning' of dispersion forces by the surface area to volume ratio difference between the two molecules.
 |
 |
| butane | methylpropane |
Geometrically speaking, the shape with the lowest surface area to volume ratio is a sphere.
Dispersion forces act at the surface of molecules, so the greater the surface area, the stronger the force (once again, all other things being equal).
A branched chain is a closer approximation to a sphere than a straight chain. Hence, straight chains have a higher boiling point than branched chain molecules, they have a larger surface area at which attractions occur.
Permanent dipole - dipole interactions
In a body of polar molecules the dipoles can attract their oppisite charges on neighbouring molecules. The partial positive charges are attracted to the partial negative charges on other molecules.

The consequence is that the molecules require larger forces to pull them apart. This means that the boiling point and the enthalpy of vaporisation is higher.
Comparison with dispersion forces
You should remember at this point that ALL molecules have dispersion forces. So any comparison must take this into account.
The best way to compare then is to use molecules that have similar relative mass. The vander Waals' forces must be similar in the two types of compound as they are a function of the relative mass. Hence any difference can be thought of as due to the permanent dipole-dipole forces.
Alkanes are examples of compounds that have only dispersion forces.
| Alkanes | Mr | b.p. /ºC | Alkanals | Mr | b.p. /ºC |
|---|---|---|---|---|---|
| ethane | 30 | -89 | methanal | 30 | -21 |
| propane | 42 | -42 | ethanal | 42 | 21 |
| butane | 54 | -0.5 | propanal | 54 | 49 |
We can see from the table that whereas the relative mass is the same between alkanes and alkanals (aldehydes), the boiling point of the alkanals is much higher. This can only be due to some other form of intermolecular force, other than dispersion force. It is of course due to dipole - dipole forces acting between the relatively negative oxygen on the carbonyl group of the alkanal and the relatively positive carbon of the carbonyl group.
Hydrogen bonding
Hydrogen bonding is a special case of dipole-dipole attractions.
When hydrogen is bonded to nitrogen, oxygen or fluorine, a very strong dipole is formed, making the hydrogen very strongly (partially) positive. This hydrogen is then attracted to the lone pairs on other similar molecules (nitrogen, oxygen and fluorine all have lone pairs) forming a hydrogen bond, which is stronger than dispersion forces or dipole-dipole interactions, but weaker than normal covalent bonding.
The reason for the strength of the positive charge on the hydrogen atoms, lies in the fact that it has only one bonding pair of electrons. Usually this pair is shared equally between hydrogen and the other bonded atom. However, in the case of hydrogen attached to oxygen, the oxygen pulls the electrons towards it leaving the hydrogen 'naked'.
As the hydrogen atom has no more electron shells, effectively it is the nucleus that is revealed. This is extremely small compared to an atom, so the developing partial charge has a much higher charge density than normal.
The group 15, 16 and 17 hydrides
The effect of hydrogen bonding on intermolecular forces can be demonstrated very well by studying the boiling points of the group 16 hydrides. As expected, the general trend is increased boiling point with increased relative molecular mass (as the dispersion force increases. However, water is completely anomalous to the trend, as it has a much higher boiling point than expected.
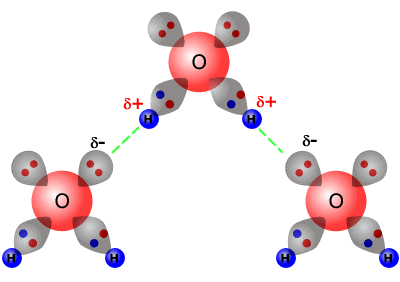 This
anomalous boiling point can be explained by the high degree of hydrogen bonding
between water molecules. Each molecule has two partially positive hydrogen
atoms and it also has two lone pairs of electrons on the oxygen atom.
This
anomalous boiling point can be explained by the high degree of hydrogen bonding
between water molecules. Each molecule has two partially positive hydrogen
atoms and it also has two lone pairs of electrons on the oxygen atom.
This means that the water molecules are able to form two hydrogen bonds per molecule with other water molecules.
The other members of the group 16 hydrides show the effect of increasing relative molecular mass on boiling point. This is to be expected, as the increase in relative molecular mass causes an increase in the strength of the dispersion forces.
Hence there is a gradual but regular increase from H2S to H2Se to H2Te
Comparing molecules
So how do we decide which is the most important force within a molecule? The answer is that we can only compare molecules in which we can 'cancel out' factors. For example, it doesn't make sense to try and compare a very large molecule with only dispersion forces, with a small molecule having hydrogen bonding. Each has a high degree of intermolecular interaction, but they arise from different sources, so can't fairly be compared.
We can, however, compare molecules with similar relative molecular mass, 'cancelling out' the dispersion influence, in which case any difference in boiling point is due to either permanent dipole-dipole interactions, or hydrogen bonding.
Example: Compare and explain the boiling points of the molecules CH3OCH3 and C2H5OH.
Methoxymethane is a slightly polar molecule, with a partial negative charge on the oxygen. Ethanol is a polar molecule with an O-H group. Both molecules have the same relative mass and so the influence of dispersion forces can be discounted in any comparison.
The boiling point of ethanol is much higher than methoxymethane, as ethanol is able to form hydrogen bonds, whereas methoxymethane has rather weaker dipole-dipole interactions.
Dimerisation
Some molecules have the ability to 'link' to other similar molecules using hydrogen bonding, in such a way that the molecules behave as two in one, so to speak. These double molecules are called dimers.
Ethanoic acid is able to form two hydrogen bonds with another ethanoic acid molecule, making a structure with double the relative molecular mass.
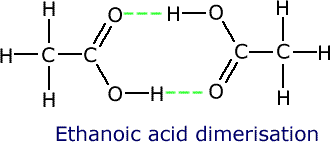
Ethanoic acid has a boiling point of 118ºC, reflecting the high apparent relative molecular mass of 120.
Solubility
The process of dissolution involves the solvent forming bonds with the solute molecules. This bond formation is exothermic, the energy being used to break the interparticular forces holding the structure together. There is also an increase in entropy when a solid dissolves, which contributes to the solubility according to Gibbs Free Energy for the process (section 4.62). If the overall Gibbs Free Energy change is negative, the substance is soluble. (remember: ∆G = ∆H - T∆S)
Substances which hydrogen bond usually dissolve in water. This is because the water molecules themselves can hydrogen bond to the solute. This bond formation makes the process favourable.
Ethanol is an alcohol which is miscible in all proportions with water. This means that it forms hydrogen bonds easily with the water. The solubility of the alcohols decreases as the relative molecular mass increases. As we ascend the homologous series of alcohols the relative mass increases by adding -CH2- units to the hydrocarbon chain.
| alcohol | formula | solubility |
|---|---|---|
| Methanol | CH3OH | miscible |
| Ethanol | CH3CH2OH | miscible |
| Propan-1-ol | CH3CH2CH2OH | miscible |
| Butan-1-ol | CH3CH2CH2CH2OH | soluble |
| Pentan-1-ol | CH3CH2CH2CH2CH2OH | less soluble |
The increasing mass means that dispersion forces become more important and the alcohol molecules begin to form intermolecular bonds with each other more easily than with the water molecules.
Worked examples - Intermolecular forces
Q243-01 As the size of the halogen molecules, X2, increases down the group, their boiling points:- Decrease due to decreasing electronegativity
- Decrease due to decreasing bond angles
- Increase due to increasing permanent dipole-dipole attractions
- Increase due to increasing permanent dispersion forces
|
Halogens are all non-polar, their intermolecular attactions are dispersion only. The force is dependent on the relative mass, which increases on descending the group. Correct response = increase due to increasing permanent dispersion forces |
Q243-02 The compounds A, B and C have approximately the same molar mass:
| A | B | C |
|---|---|---|
| C4H10 | CH3CH2CH2OH | CH3OCH2CH3 |
When the compounds are arranged in order of increasing boiling points (lowest boiling point first) the correct order is:
- A, C, B
- A, B, C
- B, C, A
- C, B, A
|
Compound A is an alkane and has no dipoles, it's intermolecular forces are dispersion only. Compound B is an alcohol, which has hydrogen bonding Compound C is an ether, which has weak permanent dipoles. The correct order is alkane, ether and then alcohol, response A, C, B |
Q243-03 Which of the compounds H2O, H2S, H2Se and H2Te has the highest boiling point?
- H2O
- H2S
- H2Se
- H2Te
|
These compounds are the hydrides of group 16. Although the relative mass increases on descending the group (increasing the dispersion forces), water, H2O, has extensive hydrogen bonding and the highest boiling point. |
Q243-04 Which intermolecular forces exist in dry ice CO2(s)?
- Covalent bonds
- Dipole - dipole attractions
- dispersion forces
- Hydrogen bonds
|
Although carbon dioxide has polar bonds, its symmetry makes it a non-polar compound. The only intermolecular forces are dispersion. |
Q243-05 The molar masses of C2H6, CH3OH, CH3F are very similar. How do their boiling points compare?
- C2H6 < CH3OH < CH3F
- CH3F < CH3OH < C2H6
- CH3OH < CH3F < C2H6
- C2H6 < CH3F < CH3OH
|
The three types of compound include an alkane, C2H6, (hydrocarbon) with only dispersion forces, an alcohol, CH3OH, with hydrogen bonding, and a halogenoalkane, CH3F, with permanent dipole - dipole attractions. The correctr order of increasing boiling point is alkane, halogenoalkane, alcohol, therefore C2H6 < CH3F < CH3OH |
Q243-06 Which sequence of Group 18 elements demonstrates a gradual decrease in the strength of the dispersion forces? All the choices are elements in the liquid state.
- Ar, Kr, Ne, Xe
- Kr, Xe, Ar, Ne
- Ne, Ar, Kr, Xe
- Xe, Kr, Ar, Ne
|
To decrease the force of dispersion attractions, the relative mass must decrease. It does so in the order Xe, Kr, Ar, Ne |
Q243-07 Which types of bonding are present in CH3CHO in the liquid state?
- I - Single covalent bonding
- II - Double covalent bonding
- III - Hydrogen bonding
- I and II only
- I and III only
- II and III only
- I, II and III
|
This question involves both intermolecular forces and intramolecular (from atom to atom within the molecule) bonding.
Correct response I and II only |
Q243-08 Why is the boiling point of PH3 lower than that of NH3?
- PH3 is non-polar whereas NH3 is polar
- PH3 is not hydrogen bonded, whereas NH3 is hydrogen bonded
- Dispersion forces are weaker in PH3 then in NH3
- The molar mass of PH3 is greater than that of NH3
|
The hydrogen atoms cannot cause hydrogen bonding unless the atom to which they are attached is highly electronegative. Thus PH3 is not hydrogen bonded, whereas NH3 is hydrogen bonded |
Q243-09 In ethanol, C2H5OH(l), there are covalent bonds, hydrogen bonds and dispersion forces. Which bonds or forces are broken when ethanol is vaporised?
- Only hydrogen bonds
- Covalent bonds and hydrogen bonds
- Covalent bonds and dispersion forces
- Hydrogen bonds and dispersion forces
|
To boil a substance all of the intermolecular forces must be broken. In ethanol there are both permanent dipole-dipole interactions (hydrogen bonding in this case) and temporary induced dipole interactions (dispersion forces). Covalent bonds are NEVER broken when a substance boils, only when it decomposes. Thus, Hydrogen bonds and dispersion forces. |
Q243-10 When the following bond types are listed in decreasing order of strength (strongest first) what is the correct order?
- Covalent < hydrogen bonds < dispersion
- Covalent < dispersion < hydrogen
- Hydrogen < covalent < dispersion
- dispersion < hydrogen bonds < covalent
|
This question is assuming that the molecules have the same relative mass, otherwise it is not possible to compare hydrogen bonds with dispersion forces. With the aforementioned assumption, the correct order is dispersion < hydrogen bonds < covalent |