|
Chemical formula are written to show the relative numbers of atoms or ions in the simplest formula unit of the compound. It helps to become familiar with the formulae of the common compounds of chemistry. This saves time during exams and helps in the general understanding of chemistry. Syllabus referenceStructure 2.1.2 - The ionic bond is formed by electrostatic attractions between oppositely charged ions.
Guidance
Tools and links
The formulae of the compounds reflect the valency rules described in section 3.22 |
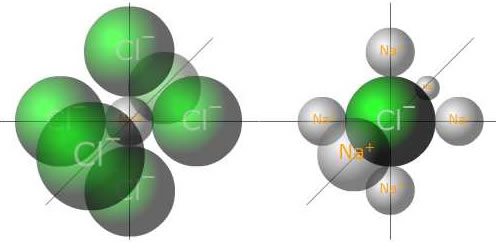
Ionic compounds have positive ions arranged in a giant lattice with negative ions. Every positive ion is surrounded by negaitive ions and every negative ion is surrounded by positive ions. The overall structure has no charge, therefore the number of positive charges is exactly cancelled out by the number of negative charges. This is also the case in the simplest formula unit.
|
|
|
Sodium ion surrounded by chloride
ions
|
Chloride ion surrounded by sodium
ions
|
| Name | formula | name | formula | |
|
metals with a valency of I+ (example sodium)
|
metals with a valency of III+ (example aluminium)
|
|||
| sodium chloride | NaCl | aluminium fluoride | AlF3 | |
| sodium nitrate | NaNO3 | aluminium nitrate | Al(NO3)3 | |
| sodium sulfate | Na2SO4 | aluminium sulfate | Al2(SO4)3 | |
| sodium sulfite (sulfate IV) | Na2SO3 | aluminium sulfite (sulfate IV) | Al2(SO3)3 | |
| sodium hydroxide | NaOH | aluminium hydroxide | Al(OH)3 | |
| sodium carbonate | Na2CO3 | aluminium carbonate | Al2(CO3)3 | |
| sodium oxide | Na2O | aluminium oxide | Al2O3 | |
|
metals with a valency of II+ (example magnesium)
|
ammonium compounds (the NH4+ ion has a valency I+) | |||
| magnesium chloride | MgCl2 | ammonium chloride | NH4Cl | |
| magnesium nitrate | Mg(NO3)2 | ammonium nitrate | NH4NO3 | |
| magnesium sulfate | MgSO4 | ammonium sulfate | (NH4)2SO4 | |
| magnesium hydroxide | Mg(OH)2 | ammonium hydroxide | NH4OH | |
| magnesium carbonate | MgCO3 | ammonium carbonate | (NH4)2CO3 | |
| magnesium oxide | MgO | ammonium dichromate | (NH4)2Cr2O7 | |
|
other compounds of interest
|
||||
| potassium manganate(VII) | KMnO4 | lead(IV) oxide | PbO2 | |
| potassium dichromate | K2Cr2O7 | manganese(IV) oxide | MnO2 | |
| potassium chromate | K2CrO4 | sodium thiosulfate | Na2S2O3 | |
In covalent compounds all of the atoms in each molecule are held to one another by bonds comprising electron pairs. In the case of double bonds there are two electron pairs involved in the bond. There are no full charges in covalent molecules unlike their ionic counterparts, but there may be partial charges caused by dipoles between atoms having different electronegativities, such as oxygen and hydrogen.
| Name | formula | name | formula | |
|
organic compounds
|
inorganic covalent compounds
|
|||
| Methane | CH4 | carbon monoxide | CO | |
| Ethane | CH3CH3 | carbon dioxide | CO2 | |
| Propane | CH3CH2CH3 | sulfur(IV) oxide | SO2 | |
| Butane | CH3CH2CH2CH3 | sulfur(VI) oxide | SO3 | |
| Ethene | CH2=CH2 |
sulfur hexafluoride |
SF6 | |
| Propene | CH2=CHCH3 | ammonia | NH3 | |
| Methanol | CH3OH | nitrogen monoxide | NO | |
| Ethanol | CH3CH2OH | nitrogen dioxide | NO2 | |
| Methanal | HCHO | phosphorus trichloride | PCl3 | |
| Ethanal | CH3CHO | phosphorus pentachloride | PCl5 | |
| Propanone | CH3COCH3 | chlorine trifluoride | ClF3 | |
| Methanoic acid | HCOOH | xenon tetrafluoride | XeF4 | |
| Ethanoic acid | CH3COOH | xenon hexafluoride | XeF6 | |
| Methyl methanoate | HCOOCH3 | hydrogen peroxide | H2O2 | |
| Ethyl ethanoate | CH3COOCH2CH3 | hydrogen chloride | HCl | |
| Chloromethane | CH3Cl | sulfuric acid | H2SO4 | |
| 1,1-dibromoethane | CH2BrCH2Br | nitric acid | HNO3 | |
| Aminoethane | CH3CH2NH2 | phosphoric acid | H3PO4 | |
| Ethanamide | CH3CONH2 | |||
| Benzene | C6H6 | |||
| Benzenecarboxylic acid | C6H5COOH | |||
| Phenol | C6H5OH | |||
| Nitrobenzene | C6H5NO2 | |||
| Phenylamine | C6H5NH2 | |||
| Glucose | C6H12O6 | |||
Ionic compounds in which water molecules has been used to build the crystal lattice (water of crystallisation) are called 'hydrated' salts
When many substances are crystallised from aqueous solution, water molecules form part of the crystal lattice and become an integral part of the final crystal structure. These molecules are called 'water of crystallisation' and when the compound is weighed out they must be taken into account.
|
Example: Cobalt(II) chloride crystals contain two molecules of water for every cobalt ion. The formula of the crystals must be written CoCl2.2H2O showing the two water molecules. Any mass of cobalt chloride weighed out also contains the water molecules. |
|
Example: Calculate the mass of copper sulfate pentahydrate, CuSO4.5H2O, that must be weighed out to prepare 1dm3 of 1 molar solution. The solution contains 1 x 1 = 1 mole of solute. Relative formula mass = 63.5 + 32 + (4 x 16) + [5 x (2 + 16)] = 249.5 Therefore 249.5g must be weighed out. |
If a compound can be prepared with or without water of crystallisation, then the terms 'hydrated' and 'anhydrous' are used to differentiate between the two types of compound.
- Hydrated copper(II) sulfate - CuSO4.5H2O
- Anhydrous copper(II) sulfate - CuSO4.H2O
Water of crystallisation can often be 'driven off' by heating the hydrated crystal, giving the anhydrous salt.
| CuSO4.5H2O blue crystals |
Some compounds form several different hydrated crystals.
Efflorescence
Certain hydrated compounds lose some of their water of crystallisation when left in the open air. This is known as efflorescence. A case in point is that of sodium carbonate decahydrate Na2CO3.10H2O. The crystals develop a powdery layer on the outside as water of crystallisation is lost to the atmosphere. Salts that effloresce cannot be used as standards for accurate preparation of solutions, as the exact composition of the crystals cannot be known.
Hygroscopy
Some compounds, typically ionic salts, absorb water from the atmosphere and increase in mass on exposure to air. Once again, the exact composition of the compound cannot be known and such salts cannot be used as primary standards. Sodium nitrate behaves in this way and, as such, is unsuitable for use in gunpowder, potassium nitrate being preferred instead.
Deliquescence
This is a special case of hygroscopy, where the salt ends up dissolving in the water absorbed from the air. A pellet of sodium hydroxide will turn into a small pool of sodium hydroxide solution when left in the air for long enough.
|
Table of some hydrated compounds
(with water of crystallisation)
|
||||
| cobalt(II) chloride | CoCl2.2H2O | dihydrate | di = 2 water molecules | |
| copper(II) sulfate | CuSO4.5H2O | pentahydrate | pent = 5 water molecules | |
| magnesium sulfate | MgSO4.7H2O | heptahydrate | hepta = 7 water molecules | |
| barium chloride | BaCl2.2H2O | dihydrate | di = 2 water molecules | |
| sodium carbonate | Na2CO3.10H2O | decahydrate | deca = 10 water molecules | |
| copper(II) chloride | CuCl2.2H2O | dihydrate | di = 2 water molecules | |
| copper(II) nitrate | Cu(NO3)2.3H2O | trihydrate | tri = 3 water molecules | |
| sodium thiosulfate | Na2S2O3.5H2O | pentahydrate | penta = 5 water molecules | |

