|
The radius of a circle or sphere is the distance from the centre to the outer edge. |
|
Definition
The atomic radius is defined as the distance from the centre of an atom to the outer edge. However, as atoms almost never exist as independent units, the measurement is taken as either the dispersion, or covalent radius, or the metallic radius.
If the element is in the solid form and the atoms are held together by dispersion forces, or covalent bonds only, as in the case of solid non-metals, then the radius is taken as one half the distance between the nuclear centres of neigbouring atoms.
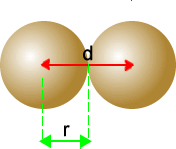 |
The distance between the two nuclear centres is given by the red line, labelled 'd'. The radius, 'r' is one half the value of the distance 'd'. |
Similary for metals, the measurement is taken as one half the distance between nuclear centres in neighbouring atoms within a metallic lattice. While definitions are consistent, size comparison may be made between atoms or other particles.
Atomic radius
Atoms consist of electrons in shells surrounding a positive nucleus. There is attraction between the electrons and the nucleus and there is repulsion between the electrons themselves.
The radius of an atom is determined by two factors.
- The number of electrons shells - the greater the number of shells the larger the atom
- The number of protons in the nucleus - the greater the number of protons in the nucleus the smaller the atom.
The number of shells is the more important of the two factors, however across a period the number of shells does not change, it is the number of protons in the nucleus that affects the radius.
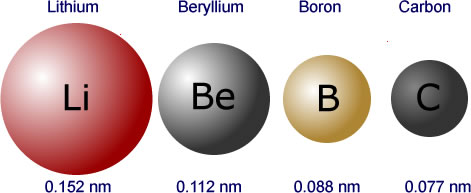 |
|
The atomic radii of the first four elements in period two shown to scale. This gives a good idea of just how much the increasing nuclear force acts on the outer energy shell. (scale 0.001 nm = 1 pixel) |

