|
Ionisation means to turn into an ion by removal of one or more electrons. The equivalent for formation of negative ions, which requires the addition of electrons, is called the electron affinity. |
Ionisation energy
"The energy required to remove 1 mole of electrons from 1 mole of gaseous atoms forming 1 mole of single charged ions".
M(g) → M+(g) + 1e-
The energy needed is dependent on the force with which the outer electron is held. This in turn is a function of the charge at the nucleus and the radius of the atom.
Electrostatic force is given by the equation:
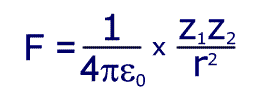
Where Z1 is the size (magnitude) if the charge on the nucleus, Z2 is the charge of an electron and 'r' is the distance between them.
Although this seems to be horribly complicated, the first section is just a constant and so the formula can be written in a much simpler way:
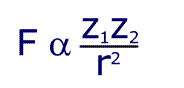
This shows that the force is proportional to the product of the charges, and inversely proportional to the square of the distance between them. Still confused? In simpler language, the further apart the charges, the weaker the force. The bigger the charge, the larger the force.
Summarised:
- Higher nuclear charge = stronger force.
- Smaller atomic radius = stronger force.
Down a group
Descending a group, the nuclear charge increases, but so does the atomic radius. These two factors would tend to cancel each other out, but the ionisation energy decreases on descending a group. This is explained by inter-electron repulsions increasing as the number of shells increases. It is sometimes called the shielding effect.
Across a period
We have already seen that the nuclear charge increases across a period and the atomic radius decreases. We would expect a steady increase in the force needed to remove an electron (the ionisation).
However, when we graph the first ionisation energies for the first 20 elements:

There is a general trend upwards across a period, but there are two points of inflexion (changes of direction) in both period 2 and 3. In period 2 the first point of inflexion is between element number 4 and 5, beryllium and boron.
There can be no doubt that the nuclear charge increases from beryllium to boron therefore the only possible reasons for boron requiring less energy to dislodge an electron are either inter-electron repulsion, or the electron is further from the nucleus that previously thought.
We now know that boron loses an electron from a 'p' sup-shell, which is more diffuse and further from the nucleus.
The point of inflexion between nitrogen and oxygen is explained by inter-electron repulsion between two electrons now paired in a 'p' orbital.
Electron affinity
The electron affinity is defined as the energy change when 1 mole of gaseous negative ions is formed from 1 mole of gaseous atoms and 1 mole of electrons. (definition not needed).
X(g) + 1e → X-(g)
The 1st electron affinity is usually exothermic (in some cases it is zero).
The electron affinity is a measure of the electron attracting ability of an atom. When the nuclear charge is high and the atomic radius is small the electron can approach closer to the atomic nucleus and feel a greater force if attraction. This translates into a larger energy release when the electron bonds to the atom.
Descending a group
The electron affinity decreases on descending a group. The number of energy shells increases and the electron is faced with more inter-electron repulsion as it approaches.
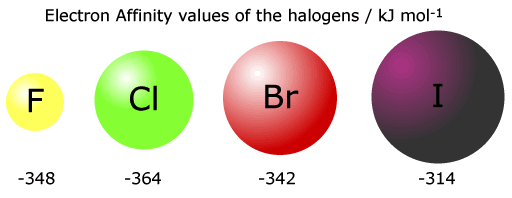
Notice that the 1st electron affinity of fluorine does not follow the group trend. This is thought to be because of inter-electron repusions in a full tightly packed second shell.
Across a period
The electron affinity increases across a period as the atomic radius gets smaller and the nuclear charge gets larger.

