|
Oxides are binary compounds formed between elements and oxygen. They may be ionic or covalent, depending on the element. Chlorides are likewise binary compounds formed between elements and chlorine. |
|
Period 3 oxides and chlorides
Oxygen is a highly reactive electronegative element that forms binary compounds easily. Chlorine is highly reactive also but it is less electronegative than oxygen and may form covalent compounds with metals of low electropositivity.
Moving from left to right across period three the elements change from metals to non-metals and the electronegativity increases. There is consequently a trend from ionic nature when combined with oxygen or chlorine towards covalent bonding. This gradual change is reflected in the melting points and boiling points of the period 3 oxides and chlorides.
 |
Sodium oxide Sodium is a highly electropositive element, which is reactive and burns easily in air or oxygen forming the oxide.
Its oxide, sodium oxide, Na2O, is a white powder with a giant ionic structure. In keeping with giant ionic structures, it has a high melting and boiling point. When molten it is a good conductor of electricity, typical of an ionic compound. Sodium chloride Sodium chloride is a white crystalline ionic compound, formed by direct combination of sodium and chlorine gas. The sodium must be first warmed before immersion in chlorine. The reaction is highly exothermic.
When molten it is a good conductor of electricity, typical of an ionic compound. |
 |
Magnesium oxide Magnesium is an electropositive reactive metal which burns easily in air or oxygen forming magnesium oxide, MgO.
Magnesium oxide is a white powdery compound with a giant ionic structure. In this case the giant ionic structure has double charged magnesium ions making its lattice relatively much stronger than that of sodium oxide. When molten it is a good conductor of electricity, typical of an ionic compound. Magnesium chloride Magnesium chloride is a white crystalline ionic solid that can be formed by burning magnesium in chlorine. Once again, it is an exothermic reaction.
When molten it is a good conductor of electricity, typical of an ionic compound. |
 |
Aluminium oxide Aluminium is a poor metal from group 13, but will burn in air at high temperatures, or when powdered, to produce aluminium oxide. The oxide has a giant ionic structure with 3+ charged aluminium ions making the lattice extremely strong.
It has a very high melting point and boiling point. When molten it is a good conductor of electricity, typical of an ionic compound. It is used in the extraction of aluminium by means of electrolysis. (Hall Cell) Aluminium chloride Aluminium chloride can be formed in the anhydrous state by heating aluminium in a stream of chlorine gas. Aluminium chloride is covalent under these conditions and sublimes from the reaction vessel and may be condensed and collected by cooling.
Note that aluminium chloride is covalent not ionic as would normally be expected from a metal and a non-metal binary compound. Aluminium is electron deficient in the structure AlCl3 and for this reason dimerises to form Al2Cl6, in which a lone pair from a chlorine on each aluminium donates into the opposite electron deficient aluminium atom. It does not conduct electricity when in the liquid state. This supports its covalent nature. |
 |
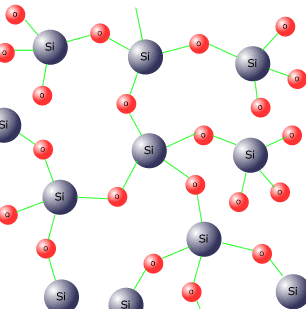
Silicon dioxide Silicon is a non-metal and both it and its oxide have giant covalent structures.
Giant covalent structures are the hardest and strongest known, hence silicon dioxide has an extremely high melting and boiling point. It does not conduct electricity when in the liquid state. This supports its covalent nature. Silicon chloride Silicon chloride is a colourless covalent liquid formed by directly heating silicon in a stream of chlorine gas at high temperature.
It does not conduct electricity when in the liquid state. This supports its covalent nature. |
 |
Phosphorous oxides Phosphorus burns readily in air forming two distinct oxides, depending on the amount of air, or oxygen, available.
Both of the oxides have simple covalent structures with low melting and boiling points. The structures consist of pyramids of phosphorus atoms linked together by bridging oxygens. In the case of phosphorus(V) oxide, there are also oxygen atoms coodinated by the lone pairs of the phosphorus atoms. They are a liquid and solid respectively at room temperature. Neither oxide conducts electricity when in the liquid state. They are covalent in nature.
Phosphorous chlorides Phosphorus forms two chlorides when directly heated in chlorine gas.
The second reaction is an equilibrium that has been studied extensively and, as such, often appears in equilibrium-type questions. Both compounds are covalent, with low melting and boiling points. Neither chloride conducts electricity when in the liquid state. They are covalent substances. |
 |
Sulfur oxides Sulfur is a yellow crystalline element that burns readily in air forming an oxide, sulfur dioxide (sulfur(IV) oxide). A second oxide, sulfur trioxide (sulfur(VI) oxide) can also be formed in excess air in the presence of a catalyst at 450ºC.
Both oxides are simple covalent compounds with low m.p. and b.p. sulfur dioxide (sulfur(IV) oxide) is a gas at room temperature. Neither oxide conducts electricity when in the liquid state. They are covalent substances. sulfur chlorides sulfur chlorides are not on the Syllabus. |
 |
Chlorine oxides Chlorine is a light green poisonous gas with a familiar smell of 'swimming pools'. It forms a series of oxides, the simplest of which is chlorine (I) oxide with the formula Cl2O, which has a simple covalent structure with a low melting and boiling point. Its higher oxide, chlorine heptoxide Cl2O7, is a covalent substance with a low melting point. It is an insulator in the liquid state. |
Summary
Bonding in the oxides and chlorides of the third period
| element | sodium | magnesium | aluminium | silicon | phosphorus | sulfur | chlorine |
| oxide | ionic | ionic | ionic | covalent | covalent | covalent | covalent |
| chloride | ionic | ionic | covalent | covalent | covalent | covalent | covalent |


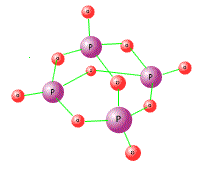
 PCl5
PCl5