|
Period 3 is the third row in the periodic table. It has eight elements beginning with sodium and ending with argon. |
Oxides
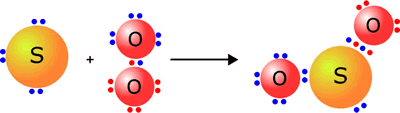
Oxides are compounds formed by elements with oxygen. Oxygen is a highly reactive element which reacts with most other elements to form binary compounds (compounds made up of only two elements), in which it usually has a valency of 2 minus, often with a release of large amounts of energy.
|
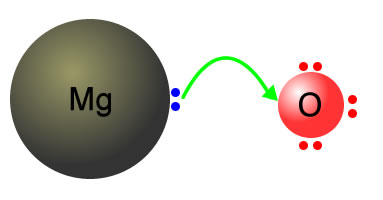
Magnesium + oxygen → Magnesium oxide Mg + O2 → 2MgO |
 |
→ |  |
|
Magnesium atoms transfer 2 electrons to the oxygen
|
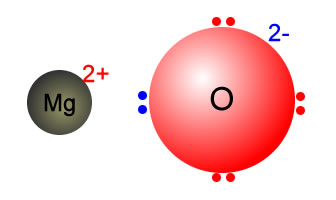
Magnesium ions are created along with oxide ions
|
Oxides are very common in nature owing to the fact that the air consists of about 20% oxygen. Many metals occur as ores containing oxides. This makes them very important economically.
- Al2O3 - Bauxite is important for the industrial production of aluminium by electrolysis (The Hall Process)
- Fe2O3 - Haematite is important for the industrial extraction of iron (The blast furnace, Bessemer Process)
Ionic and covalent oxides
Ionic bonding occurs when there is a large difference in electronegativity between two elements. Oxygen is highly electronegative and forms ionic bonds with all metals:
|
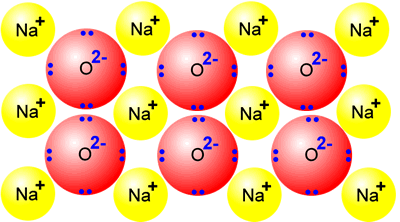
sodium + oxygen → sodium oxide 4Na + O2 → 2Na2O |
|
Sodium oxide is a typical ionic oxide. It consists of a giant structure of sodium ions and oxide ions. It displays the ionic character typical of a giant ionic structure, high melting point and electrical conductor when molten. |
 |
Covalent oxides are formed when oxygen reacts with non-metals:
|
Sulfur(IV) oxide (sulfur dioxide) is a typical simple covalent oxide. It consists of individual molecules of the formula SO2. It has typical simple covalent properties such as low melting point and electrical electrical insulator (non-conductor) when in the liquid state. |
|
Hence, there is a periodic trend from ionic oxides on the left to covalent oxides on the right.
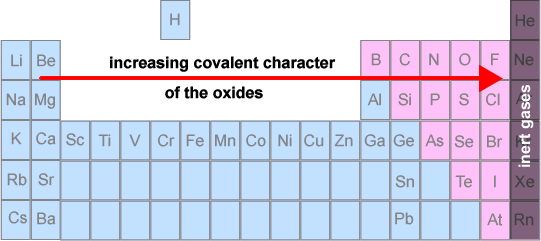
Chemical properties of period 3 oxides
 |
Sodium oxide Sodium oxide, Na2O, is a white ionic compound that reacts exothermically with water producing a solution of sodium hydroxide. It is highly basic.
|
|
|
Magnesium oxide Magnesium oxide is a white, slightly soluble, ionic compound. It reacts with water forming magnesium hydroxide, a weak base.
Magnesium hydroxide is only slightly soluble in water. |
|
|
Aluminium oxide Aluminium oxide is a white ionic compound, which is insoluble in water. It reacts with both acids and bases forming salts. This behaviour is said to be amphoteric, i.e. it behaves as both an acid and a base, it is an amphoteric oxide. With bases
With acids
|
|
|
Silicon dioxide Silicon dioxide has a giant covalent structure and is totally insoluble in water. It does react with very strong base to form salts known as silicates. This demonstrates that it is acidic oxide.
|
|
|
Phosphorus oxides Both of the phosphorus oxides are acidic and react with water forming acids. phosphorus(III) oxide reacts forming phosphoric(III) acid and phosphorus(V) oxide reacts forming phosphoric(V) acid (the stronger acid of the two).
|
|
|
sulfur oxides Both of the sulfur oxides are acidic. sulfur(IV) oxide dissolves in water forming a weak acid, whereas sulfur(VI) oxide dissolves in water forming a strong acid, sulfuric(VI) acid.
|
|
|
Chlorine oxides Chlorine forms a series of oxides with different oxidation states, not all of which are stable. In keeping with the trends outlined above, the lower oxides are weakly acidic and the higher oxides strongly acidic. Chlorine(I) oxide dissolves in water forming chloric(I) acid, a weak acid.
Chlorine(VII) oxide dissolves in water forming chloric(VII) acid, a strong acid.
|
Summary of the period 3 oxides
|
Na2O
|
MgO
|
Al2O3
|
SiO2
|
P4O10 (or P4O6) |
SO3 (or SO2) |
Cl2O7
|
|
| Adding H2O | Na2O + H2O → 2NaOH | MgO + H2O →Mg(OH)2 | Insoluble | Insoluble | P4O10 + 6H2O →4H3PO4 | SO3 + H2O →H2SO4 | Cl2O7 + H2O →HClO4 |
| Adding HCl | Na2O + H+ →2Na+ + H2O | MgO + 2H+ →Mg2+ + H2O | Al2O3 + 6H+ → 2Al3+ + 3H2O | No reaction | No reaction | No reaction | No reaction |
| Add NaOH | No reaction | No reaction | Al2O3 + 2OH- + 3H2O → 2Al(OH)4 | SiO2 + 2OH- →SiO32- + H2O | P4O10 + 12OH- →4PO43- + 6H2O | SO3 + OH- →SO42- + H2O | Cl2O7 + OH- →2ClO4- + H2O |
| Nature | Basic Oxide | Basic Oxide | Amphoteric Oxide | Acidic Oxide | Acidic Oxide | Acidic Oxide | Acidic Oxide |
| Conductivity | Good | Good | Good | None | None | None | None |
| mp / ºC | 1275 | 2852 | 2027 | 1610 | 24 | 17 | -92 |
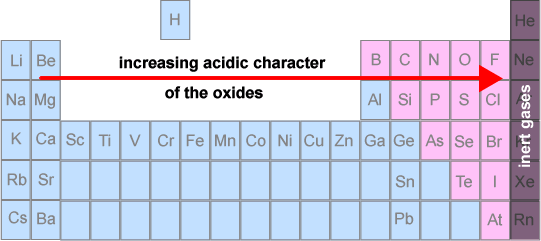
- Metal oxides are basic
- Aluminium oxide is amphoteric (reacts with both acids and bases)
- Non-metal oxides are acidic (they may also be referred to as 'acid anhydrides')
- Certain non-metal oxides do not display any acid - base character - eg N2O and CO