|
The magnetic force is one manifestation one of the fundamental forces of nature, the electromagnetic force. Magnetic fields are produces by moving electrical fields, and the force produced by a magnetic field is a vector quantity that can add to, or subtract from other magnetic forces. |
|
Paramagnetism
Paramagnetism is a relatively weak magnetic force produced by unpaired electrons. It is only detectable in the presence of an external magnetic field.
The strength of the force is directly proportional to the number of unpaired electrons in an atom or ion.
Transition metal complexes do not use the 3d orbitals in bonding, leaving them able to display paramagnetism if unpaired.
The element chromium has the electronic configuration 4s1 3d5. This means that it has six unpaired electrons and a strong paramagnetic effect.
In the form of the chromium 3+ ion it has a configuration of 4s0 3d3, and consequently less of a paramagnetic effect.
In complex ions, the actual number of unpaired electrons depends on the degree of splitting of the 3d orbitals.
Low and high spin complexes
The effect of the ligands surrounding the transition metal ion is to change the relative energies of the 3d orbitals. This is known as ligand field (or crystal field) splitting.
Different ligands have different 'strengths' in this respect and may be ordered into a spectrochemical series, with the ligands that cause the greater splitting at the top.
I- < Br- < S2- < Cl- < F- < OH-< H2O < SCN- < NH3 < CN- and CO
For example, halide ions have a weak ligand field while cyanide ligands produce a strong ligand field.
The consequences of a strong field it a greater difference in energy between the '3d' orbitals and a greater tendency for the electrons to follow Hund's rule and fill only the lower set of 3dxy, 3dxz and 3dyz orbitals. This means that there is more pairing and a weaker paramagnetic effect (if at all)
|
Example: Compare paramagnetism in the hexacyanoferrate(III) and hexaaquairon(III) complex ions. The electronic configuration of iron(III) in both ions is 4s0 3d5 However, in the hexacyanoferrate ion the octahedral ligand field splits the 3d orbitals into two groups and the strength of the ligand field means that the lower energy group preferentially fills up. This means that the lower energy group of three 3d orbitals has two filled and one single electron. Hence a paramagnetic effect due to 1 unpared electron only. The hexaaqua ion has a weaker ligand and the octahedral splitting occupies all five of the 3d orbitals. Hence, all five electrons are unpaired and there is a strong paramagnetic effect. |
Diamagnetism
Diamagnetism is a weakly magnetic effect displayed by virtually all compounds and elements. It consists of a weak force of repulsion in a magnetic field.
Transition metal compounds and elements are diamagnetic if they have no unpaired electrons.
Ferromagnetism
Ferromagnetism is the magnetic effect familiar to children. A ferromagnetic substance is able to retain its own magnetic feld
It is due to magnetic particles aligning with one another to form clusters of magnetic material called domains, whose magnetic fields add up to make a stronger field.
The more domains that are aligned together the stronger the magnetism.
The classic elements that are able to demonstrate ferromagnetism are iron, nickel and cobalt.
Nowadays there are many more materials able to retain and produce strong magnetic fields. Probably the most commercially known are the (rare earth) element neodymium magnets.
The Gouy balance
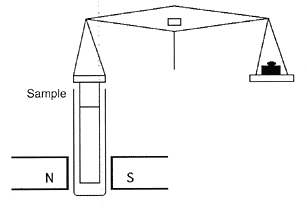 A
Gouy balance is a device that can be used to measure the weak diamagnetic
and paramagnetic effects of transition metal compounds.
A
Gouy balance is a device that can be used to measure the weak diamagnetic
and paramagnetic effects of transition metal compounds.
It can be standardised to measure the number of unpaired electrons in a complex.
It consists of a long, cylindrical sample suspended from a balance, partially entering between the poles of a strong magnet. The balance measures the apparent change in the mass of the sample as it is repelled or attracted by the region of high magnetic field between the poles.
In a practical device the whole assembly of balance and magnet is enclosed in a glass box to ensure that the weight measurement is not affected by air currents.
The sample can also be enclosed in a thermostat in order to make measurements at different temperatures

