|
All kinetics' theory emerges from data collected by experimentation. The initial aim is to measure a quantity that can be related to the concentration of a reactant or product and see how this changes in the course of reaction. |
|
Following the reaction progress
All experimental methods depend on being able to monitor a product disappearing or a reactant appearing.
Reactions that produce gases provide us with the easiest means of following a reaction's progress.
When a gas is evolved by a reaction, it may be collected, or the mass loss as the gas escapes can be recorded.
Measurement of mass loss
The experiment can easily be performed on an electronic balance and the mass recorded at suitable time intervals:
|
The mass loss method
|
|
|
|
|
The cotton wool plug allows the gas to pass through, but prevents spray from the liquid escaping as it would affect the recorded mass. As the reaction bubbles burst at the surface of the liquid they tend to produce a fine spray of liquid that could otherwise be carried out of the flask with the gas flow.
This method is more suitable when a heavy gas is evolved, such as carbon dioxide.
If the gas is very light, for example hydrogen, then large amounts would need to be released to register mass loss on the balance. If 0.01 moles of carbon dioxide are evolved, this equates to 0.01 x 44g = 0.44g, whereas for hydrogen the mass loss would be only 0.01 x 2 = 0.02g.
Gas collection methods
The volume of gas is directly related to the number of moles by Avogadro's law. If the air pressure and temperature are known then the exact number of moles of gas can be calculated using the formula: PV = nRT
|
Example: Calculate the number of moles of gas produced when 50 cm3 of gas are collected in a gas syringe at 25ºC and 1 atmosphere (100.0 kPa) pressure. PV = nRT 100.0 x 0.05 = n x 8.314 x 298 Therefore: n = 2.02 x 10-3 |
The collection method is chosen that gives the most accurate results under the given circumstances.
Gas collection 1 - The gas syringe
Gas syringes are a very convenient method of gas collection. However, the method is not without problems as they have a tendency to develop leaks rather easily over time and occasionally can stick, rendering the results unreliable.
|
Gas syringe collection
|
|
|
Another issue is that schools and colleges may not have gas syringes available.
Gas collection 2 - Displacement of water
This is probably the favoured method of gas collection in schools. Apart from being easy to set up, it is entertaining to watch!
However, if the gas is produced too rapidly then readings may be difficult to take accurately. One advantage is that the apparatus can be adapted for greater volumes of gas by substituting the burette for a large measuring cylinder.
|
Gas collection over water
|
|
|
|
|
Collection over water cannot be used when the gas in question is soluble, for example ammonia, or reacts with water.
Starting the reaction
One of the difficulties in performing rates experiments is the actual beginning of the reaction. If gases are to be collected then the reactants must be mixed with the apparatus already sealed, or gas will be lost. This may be achieved by means of what is often called a divided flask.
This is not a specific piece of equipment rather a method whereby the two reactants are kept separate until the moment desired.
|
The divided flask
|
|
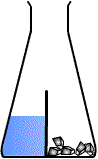 |
The flask may be divided in a number of ways depending on the experiment. The reactants are separated until the moment of mixing. The stopwatch, or timer, is started as soon as the reactants are mixed. |
The divided flask allows the whole apparatus to be sealed (gas-tight) so that no gas is lost at the beginning of the reaction.

