|
Collecting relevant data is the first step in the procedure. Once data is available and made reliable by repetition the next stage is to proceed to the data analysis. |
|
Gas collection data
Gas produced in the course of a reaction can be measured in cm3. This is directly related to the number of moles of gas according to Avogadro's law.
At room temperature this relationship is approximately 24,000 cm3 = 1 mole:

|
A graph of volume of gas (proportional to moles) against time will show the initial rate as the gradient at any time. The initial rate at t=0 is generally of more interest as this is the time when we know all of the concentrations of reactants. However, the concentrations of reactants may be calculated using simple stoichiometric relationships as shown below. |
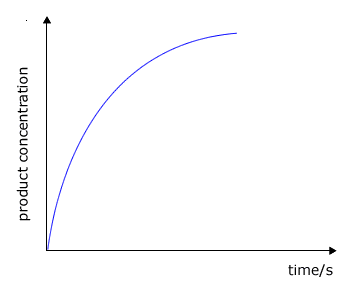 |
Reactant concentration
If the reaction equation is known then it is a routine matter to change the moles of gas into moles of reactant used up. From there, the actual concentration of reactants at different times may be calculated.
|
Example: Zinc metal produces hydrogen gas when reacted with 50cm3 sulfuric acid (1 mol dm-3) according to the equation:
Calculate the concentration of sulfuric acid after 100 seconds, if the amount of gas produced = 50cm3 Moles of hydrogen produced = 50/24000 = 2.083 x 10-3 mol. From the equation stoichiometry 1 mole of hydrogen gas is formed from 1 mole of sulfuric acid reacting. Therefore 2.083 x 10-3 of sulfuric acid has been reacted after 100s. Original moles of sulfuric acid = 0.05 x 1 = 0.05 moles Moles remaining after 100 seconds = 0.05 - 2.083 x 10-3 = 0.0479 moles Volume of acid = 50cm3 therefore molarity of acid after 100 seconds of reaction = 0.0479/0.05 = 0.0958 mol dm-3 |
Once the concentrations of the reactants have been calculated, a graph can be plotted of reactant concentration against time.
Note: If we wish to be strictly accurate, then the approximate relationship 1 mol = 24000cm3 should not be used. Instead the ideal gas equation PV=nRT must be used to calculate the moles, using the ambient temperature and pressure values.
|
The plot of concentration against time shows the rate as the gradient to the curve at any specific time. In reality we are more concerned with the initial rate, i.e. the gradient (slope) of the tangent at the steepest point to the curve. |
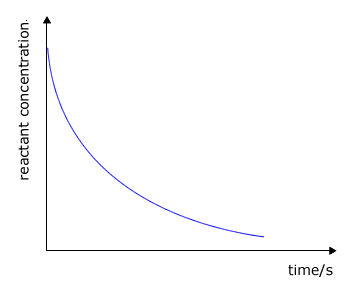 |
Mass loss data
The mass of any gas is directly related to the number of moles by the relative molecular mass of the gas.
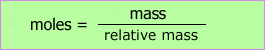
This allows us to simply plot mass of gas produced against time. Once again, the slope of the tangent to the curve gives the rate at any time.
|
The mass loss method
|
|
|
|
|
If we need to work out the concentration of any of the reactants at any specific time then we use the equation stoichiometry once again.
|
Example: 2g of calcium carbonate (marble chips) was reacted with 50 cm3 dilute hydrochloric acid according to the equation:
Calculate the concentration of hydrochloric acid after 60 seconds, if the initial concentration was 2 mol dm-3 and 0.60g of carbon dioxide was released during this time. (Mr of CO2 = 44) Moles of carbon dioxide released = 0.60g / 44 = 0.0136 mol. From the equation stoichiometry 1 mole of carbon dioxide gas is formed from 2 moles of hydrochloric acid. Therefore 0.0136 x 2 = 0.0273 moles of hydrochloric acid has reacted after 60s. Original moles of hydrochloric acid = 0.05 x 2 = 0.1 moles Moles remaining after 60 seconds = 0.1 - 0.0273 = 0.0727 moles Volume of acid = 50cm3 Therefore molarity of acid after 60 seconds of reaction = 0.0727/0.05 = 1.45 mol dm-3 |
.gif)

