|
A catalyst is a substance that affects the rate of a chemical reaction, but which can be recovered chemically unchanged at the end of the reaction. Enzymes are biological catalysts essential for the functioning of the body. |
|
Catalysts
Catalysts are substances that affect the rate of a chemical reaction and can be recovered unchanged at the end of the reaction.
It is often said that catalysts do not take part in the reaction. This would be pretty supernatural if it were the case!
A catalyst must be involved in some way, or it would not be able to inflence the processes involved in chemical reaction.
There are two modes of action of catalysts:
- heterogeneous
- homogeneous
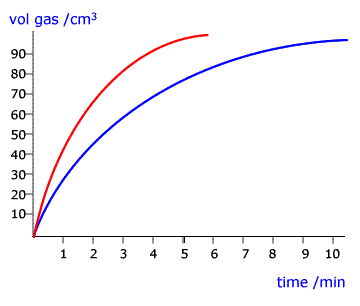
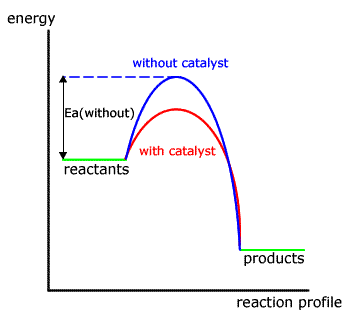
Both have the same fundamental mode of action; they lower the activation energy of the rate determining step of the reaction. This has the effect of increasing the rate of the reaction as measured in the laboratory.
|
Example: The reaction of sulfuric acid with granulated zinc.
|
Homogeneous catalysts
These are catalysts in the same state (phase - i.e. solid, liquid or gas) as the reactants.
Homogeneous catalysts speed up reactions by offering a reaction pathway of lower activation energy (and also being regenerated at the end of this process). They are often compounds that can change oxidation state easily.
A common example of a homogeneous catalyst is the acid catalysed formation of an ester. Esters are made by reacting carboxylic acids with alcohols. The condensation reaction is an equilibrium that is slow to be established at room temperature.
alcohol + carboxylic acid ![]() ester + water
ester + water
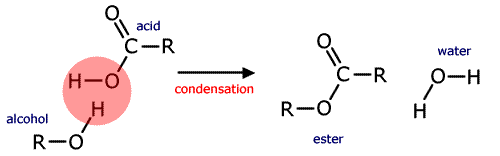
If concentrated sulfuric acid is added to the mixture the equilibrium is established much faster. (The acid also absorbs the water formed pulling the equilibrium to the right hand side).
In this reaction, the carboxylic acid, the alcohol and the sulfuric acid catalyst are all in the same phase - it is homogeneous.
Hetrogeneous catalysts
These are catalysts in a different phase (usually a solid) from the reactants.
Hetrogeneous catalysts, also called template catalysts, provide a reactive surface on which an activated complex forms, weakening the bonds and increasing the rate of collisions thus increasing the rate of reaction.
One of the reagent molecules binds to (adsorbs on) the catalyst surface forming the activated intermediate. This may then be collided with by the other reagent molecules causing reaction. The first stage of the process is the adsorption of the reagent molecule. Experience has shown that transition metals and their compounds often possess catalytic properties. It seems that the presence of 'd' orbitals is important for the binding or adsorption process.
Even though the reaction now has to pass through two steps, where originally only one was needed, both of the steps are relatively fast in comparison to the original step required. Overall the reaction is faster.
|
Example: The decomposition of hydrogen peroxide.
|
Industrial catalysts
Catalysts are very important in industry as they allow reactions to occur at a faster rate at lower temperature, making processes overall more efficient. This reflects greatly on the economics of a process.
|
process
|
catalyst used
|
catalyst type
|
| Contact (SO3) | Vanadium pentoxide | heterogenous |
| Haber (NH3) | Iron | heterogenous |
| Hydrogenation | Nickel | heterogenous |
| Polymerisation | Trialkyl titanium | heterogenous |
| Polymerisation | Oxygen | homogeneous |
Question How are catalysts found for industrial processes?
Although catalysts are used in virtually all industral processes, there is no way of predicting the suitabiity of a specific substance as a catalyst for any given reaction. Industry employs researchers to work in a fairly random way, testing out millions of different substances in order to find a suitable catalyst for a reaction. The process is arbitary and time consuming.