| The quantities of products that are released at the electrodes may be calculated by consideration of the current and time of electrolysis, as well as the species involved. In this section we look at how to perform these calculations. |
|
Relationship between current and electrical charge
As each electron possesses an electrical charge, the movement of the electrons around the external circuit may be thought of as the movement of electrical charge 'Q'. This is related to the electrical current as follows:
1 Ampere of current (I) is the passage of 1 Coulomb (C) of charge per second around the circuit. To calculate the number of coulombs of charge, it is necessary to multiply the current by the time in seconds.
| Hence:- | Q = It |
One mole of electrons has a charge equivalent to 96,500 coulombs - also called a Faraday of charge (F)
Charge on the ion
So, to release one mole of a singly charged ion at an electrode exactly one Faraday of charge (96,500 C) must pass through the electrode. If the ion has a double charge then two moles of electrons (2 Faradays) are needed to release 1 mole of ions
|
Example: Calculate the number of moles of hydrogen released when 5 amps of current passes for 3000 seconds through a solution of sulfuric acid
In the electrolysis the hydrogen gas is released at the cathode as follows: 2H+ + 2e → H2
|
|
Example: Calculate the number of coulombs needed to deposit 6.35g of copper at the cathode in an electrolysis
In electrolysis copper metal is released at the cathode as follows: Cu2+ + 2e → Cu
|
|
Example: Calculate the time that a current of 4 amps must pass to deposit 6.35g of copper. As Q = It:
|
Relative amounts of product
As ions have different charges it follows that diffierent ions will require different amounts of charge for release at an electrode. For example, copper ions have a 2+ charge, whereas silver ions have a single (1+) charge. It follows, then, that twice as many electrons are needed to deposit one mole of copper than 1 mole of silver.
|
Cu2+(aq) + 2e → Cu (s) 1 mole of copper ions + 2 moles electrons →1 mole of copper atoms |
|
Ag+(aq) + 1e → Ag (s) 1 mole of silver ions + 1 mole electrons → 1 mole of silver atom |
Therefore for the same number of moles of electrons - i.e the same electrical charge - twice as many moles of silver as copper will be deposited.
|
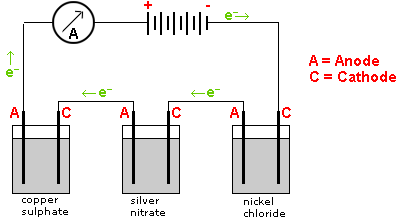
Example: In the following diagram the number of moles of silver deposited will be twice the number of moles of copper deposited. The number of moles of copper deposited will be equal to the number of moles of nickel deposited.
|