|
Haloalkanes, or halogenoalkanes, are valuable synthetic compounds, as they are one of the only types of compound that can be made directly from alkanes and the halogen atom may be easily replaced, or eliminated from the molecule. Syllabus reference Syllabus referenceReactivity 3.4.2 - In a nucleophilic substitution reaction, a nucleophile donates an electron pair to form a new bond, as another bond breaks producing a leaving group.
Guidance
Tools and links Reactivity 3.4.3 - Heterolytic fission is the breakage of a covalent bond when both bonding electrons remain with one of the two fragments formed.
Guidance
Tools and links
|
Structural features
Halogenoalkanes (also called haloalkanes) are organic molecules that contain at least one halogen atom directly attached to the carbon skeleton.
Haloalkanes have at least one halogen atom, fluorine, chlorine, bromine or iodine directly attached to the main carbon skeleton. Fluoroalkanes are particularly stable due to the strong bond formed between the carbon and fluorine atoms, and as such not typical of haloalkanes.
The halogen atom may be primary (1º), secondary (2º) or tertiary (3º) depending on the carbon atom to which it is attached.
 |
 |
 |
|
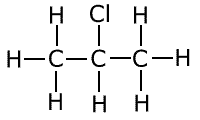
Primary halogenoalkane
|
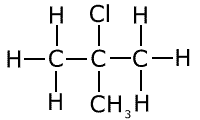
Secondary halogenoalkane
|
Tertiary halogenoalkane
|
Ensure that you understand the difference between these types of structure. A primary halogenoalkane has the halogen bonded to a carbon, which is itself only attached to one other carbon atom. A secondary halogenoalkane has the halogen bonded to a carbon that is itself attached to two other carbon atoms. In tertiary halogenoalkanes, the halogen is bonded to a carbon that is itself attached to three other carbon atoms.
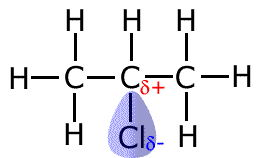
The effect of the halogen atom on the chain.
Halogen atoms are electronegative, and as such they pull or draw electron density towards themselves, and away from any carbon to which they are attached, creating polarised bonds. This leaves a partial positive charge on the carbon atom, making it a reactive center.
Substitution reactions
Halogenoalkanes are attacked by nucleophilic reagents (reagents seeking a positive charge) and undergo substitution of the halide ion by the nucleophile.
The general reaction scheme is as follows:
|
R-X + Nu- → R-Nu + X- |
Where R is an alkyl chain, X is the halide ion and Nu the nucleophile.
Reaction with hydroxide ions
Halogenoalkanes undergo nucleophilic substitution on warming with dilute alkali, making alcohols:
|
chloroethane + sodium hydroxide CH3CH2Cl + NaOH |
The hydroxide ion is the nucleophile and the chloride ion is said to be the 'leaving group'.
Nucleophilic substitution with cyanide ions, CN-
The cyanide ion, CN- is a good nucleophile and reacts with haloalkanes producing nitriles.
|
potassium cyanide + bromoethane → ethanonitrile + potassium bromide KCN + CH3CH2Br → CH3CH2CN + KBr |
This is a useful reaction for increasing the chain length by one carbon atom. Nitriles, themselves can be reduced to amines by hydrogen/nickel catalyst at 250ºC.
Mechanisms of nucleophilic substitution
Nucleophilic substitution with cyanide ions
Step 1: The cyanide ion attacks at the partially positive carbon of the dipole, making a high energy transition state.
Step 2: The bromine atom leaves with its bonding electrons as a bromide ion
Step 3. The final product is a nitrile.
Nucleophilic substitution of ammonia, NH3
Ammonia reacts with haloalkanes producing amines. The mechanism once again depends on whether the haloalkane is 1º, 2º or 3.
|
ammonia + bromoethane → ethylamine + hydrogen bromide NH3 + CH3CH2Br → CH3CH2NH2 + HBr |
Step 1: The nitrogen atom of ammonia has a lone pair that can attack the partially positive carbon, attached to the halogen atom
Step 2: The bromine atom leaves with its bonding electrons as a bromide ion
Step 3. The final product is an amine
Primary, secondary and tertiary haloalkanes
Nucleophilic substitution proceeds via different mechanisms, depending on whether the haloalkane is primary, secondary or tertiary. There are two distinct mechanisms, one for primary and one for tertiary. The mechanism followed by secondary haloalkanes is thought to be a mixture of the other two.
Primary halogenoalkanes
The nucleophile attacks the partially positive carbon that is attached to the halogen atom. This goes through a high energy transition state in which the carbon atom is associated with the incoming nucleophile as well as the outgoing halide ion.
Bimolecular Nucleophilic Substitution
Step 1: The hydroxide ion is attracted towards the partially positive carbon atom.
Step 2: A high energy transition state develops.
Step 3. The chloride ion (leaving group) breaks off leaving the hydroxide ion bonded to the alkyl group.
The mechanism for nucleophilic substitution involves two particles, the haloalkane and a nucleophile in the initial stage of reaction. For this reason it is said to be 'bimolecular'. SN2 stands for substitution - nucleophilic - bimolecular.
Tertiary halogenoalkanes
Tertiary haloalkanes react via a different mechanism. The tertiary carbonium ion formed by loss of a halide ion from the halogenoalkane is sufficiently stable to exist independently. The tertiary halalkane is in equilibrium with this carbonium ion:
(CH3)3C-Br  (CH3)3C+
+ Br-
(CH3)3C+
+ Br-
2-bromomethylpropane  tertiary carbonium ion + bromide ion
tertiary carbonium ion + bromide ion
The nuceophile can attack the tertiary carbonium ion as soon as it is formed. The rate determining step is the dissociation of the tertiary haloalkane, which only involves one species. For this reason it is said to be unimolecular. sN1
Unimolecular Nucleophilic Substitution
Step 1: The bromine atom first breaks off as an ion, leaving a tertiary carbonium ion
Step 2: The tertiary carbonium ion is attacked by the hydroxide nuclophile.
Step 3. The final product is a tertiary alcohol.
Summary of nucleophilic substitution in haloalkanes
The effect of solvent on reaction mechanism
Solvents dissolve solutes because they interact with the solute particles. There are different categories of solute depending on their structure, polarity and ability to protonate their solute particles.
Protic solvents
Protic solvents have a labile (easily released) hydrogen ion. These are usually hydrogen atoms attached to either oxygen or nitrogen.
Common characteristics of protic solvents :
- 1 solvents display hydrogen bonding
- 2 solvents have an acidic hydrogen (although they may be very weak acids such as ethanol)
- 3 solvents dissolve salts
- 4 cations by unshared free electron pairs
- 5 anions by hydrogen bonding
Examples include water, most alcohols, methanoic acid, hydrogen fluoride, and ammonia.
Aprotic solvents
Cannot donate hydrogen ions to the solute. They can however be either polar or non-polar.
Choice of solvent for nucleophilic substitution reactions
SN2 reactions are best conducted using aprotic, non-polar solvents and SN1 reactions are best conducted using protic, polar solvents.
Factors affecting reaction rate
The rate of reaction is dependent on two factors:
- Whether the haloalkane is primary, secondary of tertiary
- The actual halogen atoms attached to the alkyl chain
Effect of mechanism
Tertiary haloakanes react via an sN1 mechanism that has a much lower activation energy than the sN2 mechanism with the high energy transition state. Hence tertiary haloalkanes react faster then secondary, which in turn react faster than primary.
Effect of the halide ion
The bond fromed between carbon and iodine is much weaker than the bond formed between bromine and carbon, which in turn is weaker than the bond formed between chlorine and carbon. It is therefore easier to break off an iodide ion than a bromide or chloride ion. The rate of reaction is in the order:
iodoalkanes >> bromoalkanes >> chloroalkanes
Stereospecificity
Mechanisms that proceed via an sN2 mechanism cause inversion at the carbon atom that is attacked by the nucleophile.
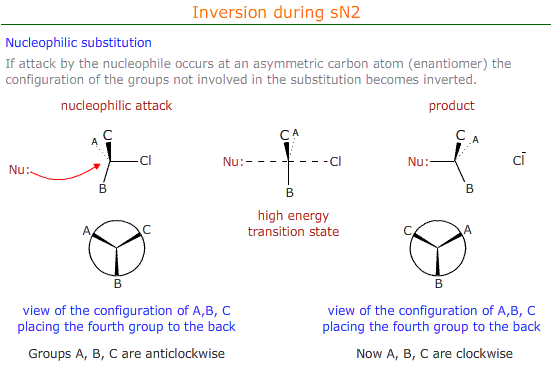
This has consequences for the optical properties and the designation of the configuration (R or S) of the stereoisomer.
If the enantiomer has an "R" conformation before nucleophilic substitution, as determined by CIP priority rules, then the product of the substitution has an "S" configuration


