Standard level
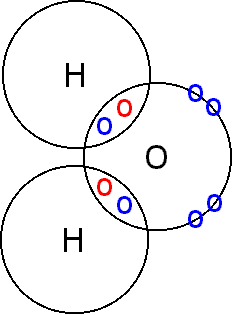
The term "property" refers to a characteristic of the bulk substance that can be observed and/or measured and which does not involve a change in chemical nature, i.e. the particles remain the same.
Background
Covalent compounds are molecular and in the liquid state the molecules are held together by forces of intermolecular attraction. These forces may be:
- • London dispersion forces (all particles)
- • Permanent dipole-dipole interactions (polar molecules only)
- • Hydrogen bonding (molecules that have hydrogen atoms attached to nitrogen or oxygen atoms)
The students will investigate the following properties of a selection of covalent liquids.
- 1. Solubility in water and non-polar solvents
- 2. Density
- 3. Boiling point
Note: The boiling point of a liquid is the temperature at which the vapor pressure of the liquid is equal to atmospheric pressure.
The ambient pressure can be found using the Phyphox app on mobile phones
Chemicals
- methanol
- ethanol
- propanone
- hexane
Apparatus
- electronic balance
- burette, 50ml
- dropping pipette
- beaker, 100ml
- analogue thermometer
- capillary tubes
- rubber ring
- beaker, 400ml
- test-tube
- Small Bunsen burner, tripod and gauze
- Organic residues disposal bottle
Density determination
- A known volume of the covalent liquid is delivered from a burette into a dry pre-weighed beaker.
- The mass of the liquid + dry beaker is then recorded.
- The covalent liquid can then be returned to the appropriate burette.
Solubility in water and a non-polar solvent
- A few drops of the covalent liquid is added to about 5 ml of water in a test tube and shaken gently
- A few drops of the covalent liquid is added to about 5 ml of methylbenzene in a test tube and shaken gently (to be disposed of in the organic residues container)
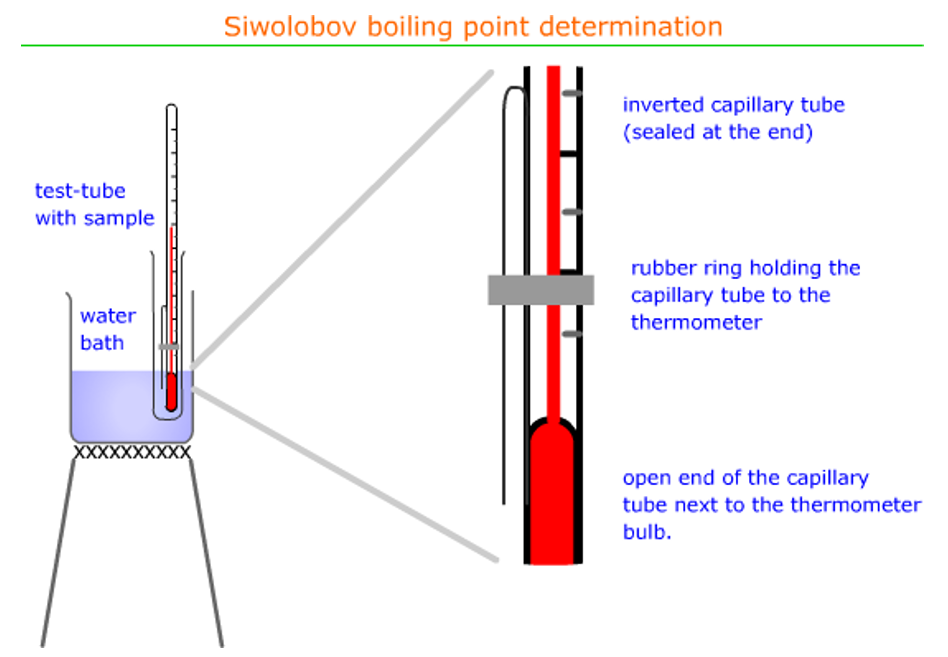
Boiling point determination (Siwolobov)
The boiling point will be determined using Siwolobov’s method
- A 10ml sample of the covalent liquid is poured into a clean, dry test-tube – the volume is not critical as the vapour pressure (and hence, the boiling point at this atmospheric pressure) is independent of everything apart from temperature.
- The capillary tube can be sealed at one end by rotating carefully in a Bunsen burner flame. Allow it to cool before continuing.
- Ensure that the rubber fixing ring is not beneath the surface of the covalent liquid.
- The water bath is pre-heated and then the test tube with sample is lowered into the hot water. It remains there until a steady stream of bubbles is observed coming out of the capillary tube. The test-tube is then removed from the water bath until the steam of bubbles stops. The temperature is recorded at this point.
- This procedure is then repeated several times until the temperature reading is steady. This is the boiling point.
- Do not forget to record the ambient pressure.
Safety
- The organic liquids are flammable and their vapours are toxic.
- Care must be taken to not inhale the vapours.
- The liquids must not be in the vicinity of a naked flame.
- Residues must be collected in an organic residues bottle, for subsequent disposal by the lab technicians.