Standard level
Colourimetry is an analytical method that depends on the absorbance of light of a specific wavelength or waveband.

Background
Copper(II) compounds are blue in colour due to the copper hexaaqua complex ion, [Cu(H2O)6]2+
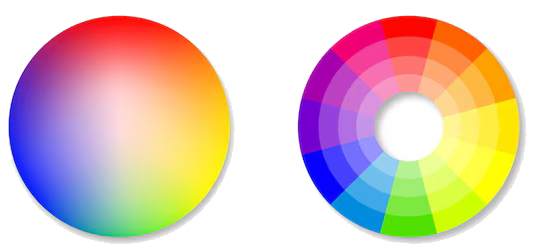
The blue colour means that the aqueous copper ions absorb more light in the orange part of the electromagnetic spectrum than in other regions. This can be shown using the complementary colour wheel that is in the chemistry databooklet.
The complementary colour wheel
The absorbance of electromagnetic radiation follows the Beer-Lambert law, where:
Absorbance = ecl
- e is the molar absorption coefficient (a constant for each solution)
- c is the concentration in mol dm-3
- l is the path length in cm
The molar absorption coefficient depends on both the absorbing species and the wavelength/waveband used.
This problem can be overcome by the use of a calibration curve prepared using known concentrations of the absorbing ion.
Chemicals
- copper(II) sulfate pentahydrate, CuSO4.5H2O
Apparatus
- Colourimeter
- Weighing boat
- Spatula
- Beaker, 250ml
- Volumetric flask, 250ml (x2)
- Pipette, 25ml
- Pipette filler
- Stirring rod
- The colourimeter must be calibrated. The instructions will depend on the model.
- Prepare a 1.0 mol dm-3 solution of copper(II) sulfate
- Use this stock solution to prepare solutions of a range of values down to 0.001 mol dm-3
- Record the absorbance of each solution using the colourimeter set to orange filter (or the nearest value).
- Plot a calibration curve of absorbance (y-axis) against concentration (x-axis).
- Use the calibration curve to determine the concentration of an unknown solution.