Standard level
Several group 1 and 2 metals burn in air releasing a large amount of energy in a highly exothermic reaction.

Background
Magnesium famously burns in air with a brilliant, hot flame that can reach up to 2000ºC.
It would be technically very difficult to collect and measure this energy, but it can be determined using Hess' law.
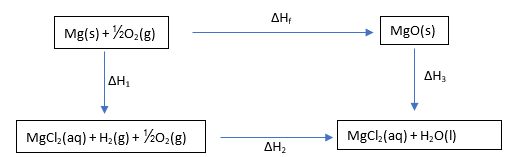
Both magnesium and magnesium oxide react with hydrochloric acid forming magnesium chloride solution.
1. The reaction of magnesium with hydrochloric acid
Mg(s) + HCl(aq) → MgCl2(aq) + H2(g)
2. The reaction of magnesium oxide with hydrochloric acid
MgO(s) + HCl(aq) → MgCl2(aq) + H2O(g)
And the energy released when hydrogen burns in oxygen is well documented
3. The reaction of hydrogen and oxygen
H2(g) + ½O2(g) → H2O(g)
We can use these three equations to produce the equation for the combustion of magnesium.
Equation 1 - equation 2
Mg(s) - MgO(s) → H2(g) - H2O(g)
Rearrange this equation to give:
Mg(s) + H2O(g) → MgO(s) + H2(g)
And now add equation 3 and rearrange:
Mg(s) + ½O(g) → MgO(s)
Hence, if we know energy values for equation 1, 2 and 3, we can determine the energy change for the combustion of magnesium.
Chemicals
- Magnesium filings
- Magnesium oxide
- Hydrochloric acid, 2 mol dm-3
Apparatus
- Polystyrene beaker and lid
- Glass beaker, 250ml
- Electronic balance
- Weighing boat
- Spatula
- Thermometer or temperature probe
- Accurately weigh out about 1g of magnesium filings (limiting reagent)
- Pour about 50cm3 of hydrochloric acid (excess) into a weighed polystrene beaker and lid
- Record the temperature of the acid
- Add the magnesium filings to the acid carefully and record the highest temperature
- Repeat twice more
- Accurately weigh out about 1.5g of magnesium oxide
- Pour about 50cm3 of hydrochloric acid into a weighed polystrene beaker and lid
- Record the temperature of the acid
- Add the magnesium oxide to the acid carefully and record the highest temperature
- Repeat twice more
This experiment could also be performed using calcium metal instead of magnesium and water instead of hydrochloric acid.
- Hydrochloric acid is corrosive.