Standard level
Increasing the temperature increases the average kinetic energy of the reacting particles.
Background
Controlling the temperature of a reaction mixture usually involves the use of a water bath to create a controllable environment.
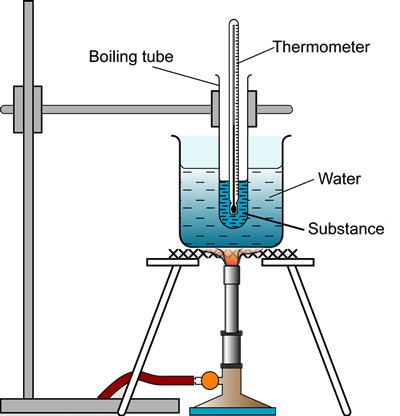
To investigate the concepts that can affect reaction rate it is important to choose reactions that have a reactant or product that can be monitored in such a way as its concentration can be determined.
Reactions that produce a gas allow the products to be monitored and measured. In this case, the decomposition of hydrogen peroxide solution.
Decomposition of hydrogen peroxide
2H2O2(aq) → 2H2O(l) + O2(g)
Chemicals
- Hydrogen peroxide(aq), 10 vol
- Manganese(IV) oxide
Apparatus
- Tripod, gauze, small Bunsen burner
- Gas syringe and delivery tube
- 10ml pipette
- Spatula
- Water bath
- Side-arm tube
- Hydrogen peroxide is an oxidising agent.
- Manganese(IV) oxide residues must be collected in the residues bottle.