Higher-level only
Chemical reactions proceed with changes in energy. This energy may either be released or absorbed.
Syllabus ref: R1.2.4Reactivity 1.2.4 - An application of Hess’s law uses enthalpy of formation data or enthalpy of combustion data to calculate the enthalpy change of a reaction. (HL)
- Calculate enthalpy changes of a reaction using ΔHf⦵ data or ΔHc⦵ data:
- ΔH⦵ = Σ (ΔHf⦵products) − Σ (ΔHf⦵reactants)
- ΔH⦵ = Σ (ΔHc⦵reactants) − Σ (ΔHc⦵products)
Guidance
- The equations to determine the enthalpy change of a reaction using ΔHf⦵ data or ΔHc⦵ data are given in the data booklet.
Tools and links
Definition
The enthalpy of reaction is the energy change when a reaction proceeds to completion in the stoichiometric amounts as expressed by the equation.
This definition may lead to confusion when the equation coefficients are greater than one. For example, in the reaction of nitrogen and hydrogen making ammonia the stoichiometric equation is:
N2(g) + 3H2(g) → 2NH3(g)
However, the enthalpy of formation of ammonia definition requires that only 1 mole of ammonia is formed
½N2(g) + ![]() H2(g)
→ NH3(g)
H2(g)
→ NH3(g)
Clearly, the energy change of formation of ammonia is half that of the enthalpy change of reaction for the balanced equation.
ΔH(reaction) = 2 x ΔHfo(NH3)
| enthalpy change | ||
| formation: | 1/2N2(g) + 3/2H2(g) → NH3(g) | -46 kJ mol-1 |
| reaction: | N2(g) + 3H2(g) → 2NH3(g) | -92 kJ mol-1 |
Reaction enthapy may be determined directly by experiment, or by using other thermodynamic data such as enthalpies of formation, or bond enthalpies.
Reaction enthalpy from enthalpy of formation values
The enthalpy of formation is: "the energy change when one mole of a substance is made from its constituent elements in their standard states"
A chemical reaction may be expressed in the following way:
Reactants → Products
In theory, if the reactants were to be changed to their elements in their standard states this would be the reverse of the enthalpy of formation, i.e. -ΔHf. The values are the same numerically but with the opposite sign.
The products are formed from these same elements, this time the energy changes are their enthapies of formation. The overall reaction is the sum of these two parts.
Reaction enthalpy = - (enthalpy of formation of the reactants) + (enthalpy of formation of the products)
∴ Reaction enthalpy = (enthalpy of formation of the products) - (enthalpy of formation of the reactants)
∑ ΔH(formation) products - ∑ ΔH(formation) reactants
Example: Find the enthalpy of reaction for the decomposition of calcium carbonate, given the following enthalpies of formation:
1 ΔHf (CaCO3) = -1207 kJ mol-1
2 ΔHf2 (CaO) = -635 kJ mol-1
3 ΔHf3 (CO2) = -394 kJ mol-1
To change CaCO3 back into its elements is the reverse of the enthalpy of formation = - (-1207 kJmol-1) = +1207 kJmol-1
2 Formation of CaO, ΔHf2 = -635 kJmol-1, and 3 ΔHf3 of CO2 = -394 kJmol-1
Therefore the enthalpy change for the decomposition = +1207 + (-635 + -394) = +178 kJ
When using this method to calculate the reaction energy, ensure that the states of the compounds given are their standard states. If this is not the case then there will be a further energy step needed.
For example, in the combustion of methane:
CH4(g) + O2(g) → CO2(g) + H2O(g)
As the equation as written, you produce water NOT in its standard state, which would be liquid at 25ºC. The equation:
Reaction energy = ∑ ΔH(formation) products - ∑ ΔH(formation) reactants
refers to the formation of water(l). To obtain water(g) from water(l) requires another endothermic process, the enthalpy of vaporisation of water.
H2O(l) →
H2O(g) ![]() ΔH(vap) = +45 kJ mol-1
ΔH(vap) = +45 kJ mol-1
|
Example: The standard enthalpy change of formation values of two oxides of phosphorus are: 1 P4(s)
+ 3O2(g) → P4O6(s) What is the enthalpy change in kJ mol-1 for the reaction shown below? P4O6(s) + 2O2(g) → P4O10(s) Equation 1 shows the enthalpy of formation of phosphorus(III) oxide (the reactant) Equation 2 shows the enthalpy of formation of phosphorus(V) oxide (the product) REM Oxygen is already an element, so its enthalpy of formation is zero by definition. ΔH(reaction) = ΔHf(products) - ΔHf(reactants) ∴ ΔH(reaction) = -3000 - (-1600) = -1400 kJ |
Reaction enthalpy from enthalpy of combustion data
Organic hydrocarbons, and compounds containing carbon hydrogen and oxygen, form the same products on complete combustion in excess air, or oxygen. The components of organic molecules, i.e. carbon and hydrogen, have known combustion values. This allows us to perform a similar treatment to that of formation enthalpies and use combustion enthalpy values to find ethalpies of reaction between two organic compounds.
For example if we wish to find the enthalpy change of the reaction:
C2H2(g) + H2(g) →
C2H4(g)![]() ΔH = ?
ΔH = ?
It would be impossible to carry out the experiment in practice as the reaction could never be made to stop at ethene; a mixture of products would always ensue.
However, combustion of the reactants gives two moles of carbon dioxide and two moles of water:
C2H2(g) + O2(g) →
2CO2 + H2O(g)![]() ΔH = -1301 kJ
ΔH = -1301 kJ
H2(g) + ½O2(g) →
H2O(l)![]() ΔH
= -268 kJ
ΔH
= -268 kJ
H2O(l) →
H2O(g)![]() ΔH
= +41.1 kJ
ΔH
= +41.1 kJ
This is exactly the same result as complete combustion of the products:
C2H4(g) + 1½O2(g)
→ 2CO2 + 2H2O(g)![]() ΔH = -1411 kJ
ΔH = -1411 kJ
Hence if we imagine that the combustion products are a kind of stepping stone to the reactants, we have the combustion of the reactants to get to carbon dioxide and water, followed by the reverse of the combustion of the products to go from carbon dioxide and water to the actual products themselves.
The enthalpy change is given by:
| Reaction enthalpy = combustion enthalpy of the reactants - combustion enthalpy of the products |
Reaction enthalpy from bond enthalpy data
This is covered in detail under bond enthalpies in section 4.42
Significance of the ΔH sign
If the sign of the enthalpy change of reaction is positive then it is an endothermic reaction. In other words a reaction that absorbs, or needs energy to proceed. In the example above, the decomposition of calcium carbonate would be expected to be endothermic, as the compound has to be broken apart. This required energy (bond breaking).
A negative sign, on the other hand, indicates that energy is released, i.e. it is an exothermic reaction. In the example below, ammonia formation, -46 kJ of energy is released for every mole of ammonia formed.
½N2(g) + 1½H2(g)
→ NH3(g)![]() ΔH
= -46 kJ
ΔH
= -46 kJ
Worked examples
Q423-01 According to the enthalpy level diagram below, what is the sign for ΔH and what term is used to refer to the reaction?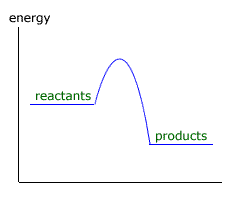
| ΔH | reaction | |
| A. | positive | endothermic |
| B. | negative | exothermic |
| C. | positive | exothermic |
| D. | negative | endothermic |
|
The chemical energy of the reactants decreases, therefore ΔH is negative. This is an exothermic reaction. |
Q423-02 An equation for a reaction in which hydrogen is formed is:
| CH4 + H2O → 3H2 + CO ΔH = +210 kJ |
Which energy change occurs when 1 mole of hydrogen is formed in this reaction?
- 70 kJ of energy are absorbed from the surroundings
- 70 kJ of energy are released to the surroundings
- 210 kJ of energy are absorbed from the surroundings
- 210 kJ of energy are released to the surroundings
|
The energy change is shown for production of 3 moles of hydrogen, hence the energy change for 1 mole of hydrogen would be +210/3 = +70 kJ |
Q423-03 Which statements are correct for an endothermic reaction?
- I - The system absorbs heat
- II - The enthalpy change is positive
- III - The bond enthalpy total for the reactants is greater than for the products
- I and II only
- I and III only
- II and III only
- I, II and III
|
Endothermic reactions absorb heat from the surroundings, they have a positive enthalpy change, AND the bond enthalpy total for the reactants is greater than that of the products, as more energy had to be put in to break the reactants bonds than was released by forming the products bonds. |
Q423-04 When the solids Ba(OH)2 and NH4SCN are mixed a solution is produced and the temperature drops.
Ba(OH)2(s) + 2NH4SCN(s) → Ba(SCN)2(aq) + 2NH3(g) + 2H2O(l)
Which statement about the energetics of this reaction is correct?
- The reaction is endothermic and ΔH is negative
- The reaction is endothermic and ΔH is positive
- The reaction is exothermic and ΔH is negative
- The reaction is exothermic and ΔH is positive
|
The temperature drops so the reaction is endothermic. Endothermic reactions have positive enthalpy change. |
Q423-05 Which statement about exothermic reactions is not correct?
- They release energy
- The enthalpy change (ΔH) is negative
- The products have a greater enthalpy than the reactants
- The products are more stable than the reactants
|
Exothermic reactions release energy ot the surroundings, the products are more stable than the reactants and ΔH is negative. Response D |
Q423-06 Which statement is correct for an endothermic reaction?
- The products are more stable than the reactants and ΔH is positive.
- The products are less stable than the reactants and ΔH is negative.
- The reactants are more stable than the products and ΔH is positive.
- The reactants are less stable than the products and ΔH is negative.
|
Endothermic reactions have products that are less stable than the reactants, ΔH is positive. Response C |
Q423-07 When solid ammonium nitrate, NH4NO3(s) is dissolved in water the temperature decreases. Which statement about the dissolution process of ammonium nitrate in water is correct?
- It is endothermic with ΔH greater than zero.
- It is endothermic with ΔH less than zero.
- It is exothermic with ΔH greater than zero.
- It is exothermic with ΔH less than zero.
|
The temperature decreases so it's an endothermic reaction. This means that ΔH is positive, i.e. greater than zero. Response A |
Q423-08 Which statement about this reaction is correct?
2Fe(s) + 3CO2(g) → Fe2O3(s) + 3CO(g) ΔH = +26.6 kJ
- 26.6 kJ of heat are released for every mole of Fe reacted.
- 26.6 kJ of heat are absorbed for every mole of Fe reacted.
- 53.2 kJ of heat are released for every mole of Fe reacted.
- 13.3 kJ of heat are absorbed for every mole of Fe reacted.
|
The heat of reaction is for 2 moles of iron reacted, therefore for 1 mole of iron reacted, the enthalpy change is exactly one half (endothermic = energy absorbed) = +13.3 kJ |
Q423-09 Excess thionyl chloride, SOCl2, can be removed from a reaction mixture by reacting it with water according to the equation:
SOCl2(l) + H2O(l) → 2HCl(g) + SO2(g)
Use the following data to calculate ΔH for this reaction.
| SOCl2(l) | H2O(l) | HCl(g) | SO2(g) | |
| H f(kJ mol-1) | - 245.6 | - 285.8 | - 92.3 | - 296.8 |
- - 142.3
- - 50.0
- + 50.0
- + 142.3
|
The enthalpy of formation is defined as the energy change when 1 mole of a substance is formed from its constituent elements in their standard states. Each component in an equation has a corresponding enthalpy change of formation. The compoounds on the reactants side must be reversed into their original elements before going on to make the products on the right hand side. The energy change obtaining the elements from the reactants on the left hand side is the negative of the enthalpies of their formation (i.e. the opposite sign). The energy of formation of the products can then simply be added to this. -[ΔH -[ - 245.6 - 285.8] + 2(- 92.3) - 296.8 = ΔH (reaction) 531.4 - 184.6 - 296.8 = ΔH (reaction) Therefore ΔH (reaction) = + 50 kJ |
Q423-10 What is ΔH for the reaction below in kJ?
CS2(g) + 3O2(g) → CO2(g) + 2SO2(g)
[ΔHf /kJ mol-1; CS2(g): 110, CO2(g): -390, SO2(g): -290]
- -570
- -790
- -860
- -1080
|
Reaction enthalpy = formation enthalpy of the products - formation enthalpy of reactants Reaction enthalpy = [-390 + (2 x -290)] - [110 + 0] = -1080 kJ |
| Now test yourself |