Standard level
Syllabus ref: R1.3.2Reactivity 1.3.2 - Incomplete combustion of organic compounds, especially hydrocarbons, leads to the production of carbon monoxide and carbon.
- Deduce equations for the incomplete combustion of hydrocarbons and alcohols.
Guidance
Tools and links
- Inquiry 2 - What might be observed when a fuel such as methane is burned in a limited supply of oxygen?
- Reactivity 2.1 - How does limiting the supply of oxygen in combustion affect the products and increase health risks?

Incomplete combustion
This means that the combustion reaction has not gone as far as it could in terms of releasing energy. Not all of the reactant has thoroughly reacted and, in the case of an organic molecule, not all of the carbon has turned to carbon dioxide.
As hydrocarbons are used for fuels, incomplete combustion is both inefficient and dirty, in the sense that the products may be solid carbon (smoke) or carbon monoxide, CO, a harmful odourless colourless gas.
Note: all of the hydrogen contained in a fuel produces water - there are no exceptions.
Fuels that burn efficiently do not release much visible light during combustion - the flame may even appear virtually invisible. This is the case for combustion of the lower relative mass alcohols such as methanol and ethanol in plentiful air.
If the air supply is restricted, for example by using a spirit burner with alcohol, then some of the fuel burns inefficiently forming carbon microparticles that glow in the heat of the flame giving the flame a yellow colour.
Particulate Matter
When hydrocarbon fuels burn incompletely, the carbon that is produced makes tiny particles of solid carbon suspended in the air. These tiny particles of carbon are called microparticulates. They can be any size and are known to be harmful to the lungs where they act as foci for the formation of localised damage and possible lung cancer.
When the particles are large, we can see them as smoke, which normally settles out of the air onto the surface. However, smaller microparticulates may remain suspended in air for a very long time. Most governments now monitor these materials using a crude measurement system of PM10, PM2.5 etc. To indicate microparticular level with particles that measure on the micrometre scale.
PM10 measures all of those particles with a diameter of 10 micrometres or less, 1 x 10-5m.
Worldwide, exposure to PM2.5 contributed to 4.1 million deaths from heart disease, stroke, lung cancer, chronic lung disease, and respiratory infections in 2016. Overall, ambient particulate matter is one of the leading risk factor for premature death globally. [ref Wikipedia]
Carbon monoxide
Carbon monoxide is formed when hydrocarbon fuels are burned in insufficient air for complete combustion. The gas is colourless, odourless and highly toxic.
Carbon monoxide is a good ligand for transition metal ions, forming a coordinate bond to the ion using the lone pair of electrons on the carbon atom.
Haemoglobin has an iron atom at the centre of a porphyrin structure that is used to capture oxygen from the lungs and transport it around the body in the bloodstream.
Carbon monoxide coordinates to this iron atom in the +2 oxidation state) forming carboxyhaemoglobin, which renders the haemoglobin incapable of oxygen transport. The brain becomes starved of oxygen resulting in unconsciousness and death.
Incomplete combustion is the reason why all hydrocarbon fuel domestic boilers must be checked on a regular basis to ensure that the air flow is sufficient to prevent the build up of deadly carbon monoxide gases in the home.
Equations for incomplete combustion
There are no specific equations for incomplete combustion, as there are many ratios of reactants from which products can be formed.
Taking the simplest hydrocarbon, methane, we can propose several possible incomplete combustion equations, simply by varying the ratio of methane to oxygen.
Complete combustion of methane
CH4(g) + 2O2(g) → CO2(g) + 2H2O(l)
Remember that all of the hydrogen MUST become water, so we need at least one molecule of oxygen
If there is less oxygen than is needed for complete combustion then carbon monoxide and/or carbon is formed. Any ratio of methane to oxygen can be selected for incomplete combustion providing that the amount of oxygen is less than that required for complete combustion.
Incomplete combustion of methane
2CH4(g) + 3O2(g) → 2CO(g) + 4H2O(l)
Another possible equation:
Incomplete combustion of methane
2CH4(g) + 3O2(g) → C(s) + CO2(g) + 4H2O(l)
It is the same story for every other fuel.
Pollution from cars
Internal combustion engines mix air with petrol vapour and explode it using a spark inside a restricted space, a cylinder. The resultant explosion pushes the piston back up the cylinder and causes the crank shaft to rotate, driving the car wheels via gears.
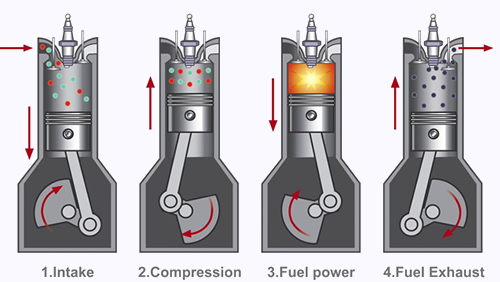
The petrol-air mixture must be just right for a powerful explosion, while reducing the amount of incomplete combustion products. The petrol and air are mixed together before injection into each cylinder.
Petrol is mostly 2,2,4-trimethylpentane, C8H18, which burns incompletely at the most favourable ratio forming both carbon monoxide and carbon dioxide (as well as water).
A possible equation for the combustion of petrol
C8H18(g) + 12O2(g) → CO(g) + 7CO2(g) + 9H2O(l)
There are millions of cars burning petrol in the world using internal combustion, meaning that carbon monoxide is a common pollutant gas, particularly in cities.
Car manufacturers incorporate catalytic converter in the car exhaust system to reduce this carbon monoxide pollution.
The catalytic converter makes use of another pollutant gas produced in the engine, nitrogen monoxide, NO, and uses a platinum/palladium/rhodium alloy to catalyse the reaction:
Catalytic converter reaction
2CO(g) + 2NO(g) → 2CO2(g) + N2(g)
The catalytic converter helps to reduce the amount of these two pollutant gases. However, the convertion is not 100% efficient and also unburnt hydrocarbons and microparticulates are released via the exhaust, making cars a major pollution source in cities.

Worked examples
Q461-01 Which of the changes below occurs with the greatest increase in entropy?- Na2O(s) + H2O(l) → 2Na+(aq) + 2OH-(aq)
- NH3(g) + HCl(g) → NH4Cl(s)
- H2(g) + I2(g) → 2HI(g)
- C(s) + CO2(g) → 2CO(g)
|
Entropy can be considered the degree of disorder of a chemical system. It is increased by the number of particles and their temperature. In this case it is important to examine the number of moles of free particles on both sides of the equation. It may be seen that in equation D there are more moles of gas (maximum entropy) on the right hand side than on the left hand side. Thus the entropy increases from left to right. correct response Although there are more free ions in A this is not as important in entropy terms as an increase in the number of moles of gas. In equation B there is a large decrease in entropy (two gases make a solid) and in equation C the number of moles of gas on both sides is equal. |
Q461-02 In which of the following reactions is the entropy change ( S) closest to zero
- SO2(g) + ½O2(g) → SO3(g)
- Br2(l) → Br2(g)
- H2(g) + I2(g) → 2HI(g)
- 3Ca(s) + N2 → Ca3N2(s)
|
Entropy can be considered the degree of disorder of a chemical system. It is increased by the number of particles and their temperature. In this case it is important to examine the number of moles of free particles, i.e. gas, on both sides of the equation. Equation A the moles of gas decreases from reactants to products, ΔS is negative. Equation B the moles of gas increases from 0 to 1, ΔS is positive. Equation C the moles of gas stays the same from reactants to products, ΔS = 0. correct response Equation D the moles of gas decreases from 1 to 0, ΔS is negative. |
Q461-03 Estimate, without doing a calculation, the magnitude of the entropy change for the following reaction.
Fe2O3(s) + 2Al(s)  2Fe(s) + Al2O3(s)
2Fe(s) + Al2O3(s) |
|
Examination of the equation reveals that the compounds on both sides of the equation are in the solid state. As solids have very low entropy it is safe to estimate that the entropy difference between reactants and products is negligible. Hence ΔS = 0. |
Q461-04 Consider the following reaction:
| N2(g) + 3H2(g) → 2NH3(g) |
The absolute entropy values, S, at 300K for N2(g), H2(g)
and NH3(g) are 193, 131 and 192 JK-1 mol-1
respectively. Calculate ΔSo
for the reaction and explain the sign of So.
|
On the left hand side there is one mole of nitrogen and three moles of hydrogen. Their entropy = 193 + (3 x 131) = 586 JK-1 On the right hand side there are two moles of ammonia. Entropy = (2 x 192) = 384 JK-1 The entropy change, ΔS The negative sign indicates that the entropy has decreased from reactants to products. |
Q461-05 Which reaction has the greatest positive entropy change?
- CH4(g) + 1½O2(g) → CO(g) + 2H2O(g)
- CH4(g) + 1½O2(g) → CO(g) + 2H2O(l)
- CH4(g) + 2O2(g) → CO2(g) + 2H2O(g)
- CH4(g) + 2O2(g) → CO2(g) + 2H2O(l)
|
A positive entropy change means that the products have more entropy than the reactants. Gases have the largest entropy values, therefore we are looking for the reaction that produces the greatest positive change in moles of gas. reaction 1 2½ moles gas → 3 moles of gas. An increase by ½ mole gas correct response reaction 2 2½ moles gas → 1 mole of gas. A decrease of 1½ mole gas reaction 3 3 moles gas → 3 moles of gas. No change in moles reaction 4 3 moles gas → 1 mole of gas. A decrease of 2 moles of gas |
Q461-06 Which reaction occurs with the largest increase in entropy?
- Pb(NO3)2(s) + 2KI(s) → PbI2(s) + 2KNO3(s)
- CaCO3(s) → CaO(s) + CO2(g)
- 3H2(g) + N2(g) → 2NH3(g)
- H2(g) + I2(g) → 2HI(g)
|
An increase in entropy change means that the products have more entropy than the reactants. Gases have the largest entropy values, therefore we are looking for the reaction that produces the greatest positive change in moles of gas. reaction 1 0 moles gas → 0 moles of gas. No change in moles of gas reaction 2 0 moles gas → 1 mole of gas. A increase of 1 mole of gas correct response reaction 3 4 moles gas → 2 moles of gas. A decrease of 2 moles of gas reaction 4 2 moles gas → 2 mole of gas. No change in moles of gas |
Q461-07 Some chlorine gas is placed in a flask of fixed volume at room temperature. What change will cause a decrease in entropy?
- Adding a small amount of hydrogen
- Adding a small amount of chlorine
- Cooling the flask
- Exposing the flask to sunlight
|
Anything that increases the disorder, such as mixing two gases, or increasing the temperature, increases the entropy. The reverse is also tru. Hence decreasing the temperature decreases the entropy, e.g. Cooling the flask |
Q461-08 Which reaction has the largest positive value of ΔS
- CO2(g) + 3H2(g) → CH3OH(g) + H2O(g)
- 2Al(s) + 3S(s) → Al2S3(s)
- CH4(g) + H2O(g) → 3H2(g) + CO(g)
- 2S(s) + 3O2(g) → 2SO3(g)
|
An increase in entropy change means that the products have more entropy than the reactants. Gases have the largest entropy values, therefore we are looking for the reaction that produces the greatest positive change in moles of gas. reaction 1 4 moles gas →
2 moles of gas. A decrease of 2 moles of gas, ΔS reaction 2 0 moles gas →
0 mole of gas. No change in moles of gas, ΔS reaction 3 2 moles gas →
4 moles of gas. An increase of 2 moles of gas, ΔS reaction 4 3 moles gas →
2 mole of gas. A decrease of 1 mole of gas, ΔS |
Q461-09 Which equation represents a change with a negative value for ΔS?
- 2H2(g) + O2(g) → 2H2O(g)
- H2O(s) → H2O(g)
- H2(g) + Cl2(g) → 2HCl(g)
- 2NH3(g) → N2(g) + 3H2(g)
|
A negative value for ΔS means that the products have less entropy than the reactants. There are fewer moles of gas in the products than in the reactants. reaction 1 3 moles gas → 2 moles of gas. A decrease of 1 moles of gas, ΔS = negative correct response reaction 2 0 moles gas → 1 mole of gas. An increase by 1 mole of gas, ΔS = positive reaction 3 2 moles gas → 2 moles of gas. No change in moles of gas , ΔS = 0 (approx) reaction 4 2 moles gas → 4 mole of gas. An increase by 2 moles of gas, ΔS = positive |
Q461-10 Which change does not lead to an increase in entropy?
- Mixing nitrogen and oxygen gases at room temperature
- Cooling steam so that it condenses to water
- Heating hexane to its boiling point
- Dissolving sugar in water
|
Entropy is increased by:
From the choices given, only cooling steam reduces the entropy of the system |