Standard level
Both increasing concentration and pressure brings more particles into collision proximity.
Syllabus ref: R2.2.3Reactivity 2.2.3 - Factors that influence the rate of a reaction include pressure, concentration, surface area, temperature and the presence of a catalyst.
- Predict and explain the effects of changing conditions on the rate of a reaction.
Guidance
Tools and links
- Tool 1 - What variables must be controlled in studying the effect of a factor on the rate of a reaction?
- Nature of science, Tool 3, Inquiry 3 - How can graphs provide evidence of systematic and random error?
Concentration
Concentration is a measure of the number of particles per unit volume, measured in moles per unit volume. The mole is a large number of particles (an Avogadro number = 6.02 x 1023). The units of concentration are written as mol dm-3.
More particles moving in the same volume results in more collisions, so an increased concentration normally brings about an increase in the rate.
REM Solids cannot have a concentration;
their particles are not free to move, unlike particles in solutions and gases.
Example: Which of the following changes would result in a greater increase in rate of reaction when 2g of magnesium ribbon reacts with 200 cm3 of 2 mol dm-3 hydrochloric acid.
- Adding 2g of magnesium ribbon
- Changing the concentration of HCl to 4 mol dm-3
Although increasing the mass of magnesium does increase the rate (as there is more surface area available for reaction), solids do NOT have a concentration as their particles are not free to move.
Increasing the concentration of the HCl makes many more hydrogen ions available for reaction by collision and this has by far the greater effect on the rate.
The exception to greater concentration increasing the rate, is when the order with respect to a component is zero, in which case it is not implicated in the rate determining step of the reaction (see 6.34: mechanisms) and increasing its concentration does not increase the rate.
Pressure
Is directly proportional to the concentration of particles in a gas. The particles collide with the external walls of the container and exert a pressure. To increase the pressure of a fixed mass of gas there are only two possible approaches:
- The volume can be reduced
- The temperature can be increased
In the first instance, there are more particles within a specific volume, more collisions with the walls of the container and each other.
In the second case the particles move faster and therefore collide more often (and with greater average energy).
Increasing the pressure and consequently the number of collisions increases the rate of reaction.
Beware of ascribing increased collisions as the reason for increased rate as the temperature rises. Although there are more collisions, it is the effectiveness of the collisions that is important in this case.
Once again, the exception to greater pressure increasing the rate, is when the order with respect to a component is zero and it is not implicated in the rate determining step of the reaction (see 6.34: mechanisms), in which case increasing pressure does not increase the rate.
Partial Pressure
The pressure exerted by one gaseous component of a mixture is called the partial pressure with respect to that specific component. The total pressure of a gas equals the sum of the partial pressures. For a mixture of 3 gases:
Total pressure = partial pressure (gas 1) + partial pressure (gas 2) + partial pressure (gas 3)
P(tot) = P(gas 1) + P(gas 2) + P(gas 3)
The partial pressure of a component is also equal to the mole fraction of that component multiplied by the total pressure. For a mixture of two gases, A and B:
Partial pressure (gas A) = Mole fraction (gas A) x Total pressure
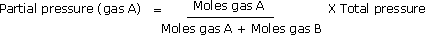
Increasing the partial pressure of a gas in a gaseous reaction mixture generally causes a corresponding increase in the rate of the reaction, providing that the reactant appears in the rate expression.