Standard level
Particles in any sample of matter have energy spread out in a random fashion. This distribution follows the statistical patterns first described by Maxwell and Boltzmann.
This is important in reaction kinetics as only particles with high enough energy can cause reaction to occur when they collide.
By understanding this distribution and how it varies under different situations we can better understand how the rate of chemical reactions changes.
Syllabus ref: R2.2.4Reactivity 2.2.4 - Activation energy, Ea, is the minimum energy that colliding particles need for a successful collision leading to a reaction.
- Construct Maxwell–Boltzmann energy distribution curves to explain the effect of temperature on the probability of successful collisions.
Guidance
Tools and links
Distribution of energy
The distribution of energy in a sample of particles is shown by the Maxwell-Boltzmann curve.
The curve is arrived at by statistical analysis.
Statistics and probability tells us that if the energy is distributed randomly over a large number of particles there will be a much greater probability that some arrangements are found rather than others.
The Maxwell Boltzmann curve shows us that are few particles with small amounts of energy and few particles with large amounts of energy. The majority of the particles possess an intermediate amount of energy.
Increasing the temperature flattens the curve as more particles attain higher energies and the median energy (the hump) moves to the right (higher energy) side.
Activation energy
For chemical reaction to occur bonds must be broken and formed. The energy required to break the bonds comes from the collision. The activation energy is the minimum amount of energy required for reaction to occur between colliding particles.
Not all collisions between suitable particles are going to result in successful reaction. There are some collisions that simply do not have enough energy to break bonds. The collisions in which the particles do have enough energy for bond breaking can react. The activation energy of a reaction is given the symbol Ea.
In a reaction profile the activation energy is the highest energy step taken during the course of the reaction. (Note: there may be several activation energy steps in a multi-step reaction)
The activation energy of a reaction is a constant for that specific reaction - it can only be changed if the mechanism of the reaction itself changes. This occurs during catalysis.
Effect of temperature
The shape of the Maxwell Boltzmann curve is temperature dependent. As the temperature increases there are more particles with higher energy than at a lower temperature. The shape of the curve broadens and flattens (it must flatten if it gets broader, as the total number of particles cannot change).
We can see from the curves that there are more particles with higher energy at a higher temperature. This means that there will be more successful colisions, i.e. collision with the required activation energy.
For this reason, increasing the temperature of a reaction increases the rate. As a rough guide, the rate doubles for every ten degree increase in temperature.
It is tempting to say that the reason temperature affects rate is that there are more collisions, due to the fact that the particles are travelling faster. While it is true that there are more collisions, this factor is much less important than the activation energy factor.
Collision geometry - the orientation factor
When two cars collide the degree of damage depends on the angle with which they collide. Likewise, a car that reverses into a tree is not going to break its headlights!
Taking these things to a logical conclusion, when two particles collide the orientation of the collision is important for two reasons:
- The collision must occur with enough energy to result in reaction
- The particles must collide in such a way as to break the required bonds.
The second of these collision requirements is called the orientation factor. Not all collisions result in reaction, even if the combined energy of the particles is theoretically large enough, because the orientation is incorrect.
Catalysis
A catalyst is a substance that increases the rate of a chemical reaction without itself getting consumed in the reaction. A catalyst can be recovered chemically unchanged at the end of the reaction.
Catalysts work by offering a different route to the products (mechanism) with a lower activation energy.
The consequence is that there are now more particles at a given temperature that have the required activation energy to react.
Worked examples
Q224-01 The rate of a chemical reaction approximately doubles when its temperature is raised from 25ºC to 35ºC because- the fraction of molecules with energy greater than the activation energy has increased dramatically.
- the activation energy for the reaction has been lowered.
- a very few molecules have energy greater than the activation energy.
- all the molecules have a little more energy.
|
Using the Maxwell - Boltzmann curve we see that, at higher energy, the curve becomes broader and flatter, and the peak moves to the right hand side. This means that the total number of particles having more than any given energy, for example the activation energy, increases rapidly and by large amounts as the temperature rises. Therefore the fraction of molecules with energy greater than the activation energy has increased dramatically |
Q224-02 The enthalpy change for the formation of one mole of nitrosyl chloride, ΔH = -38 kJ.
NO(g) + ½Cl2(g) → NOCl(g)
The activation energy for this reaction is 62 kJ. The activation energy for the reverse reaction is:
- 38 kJ.
- 62 kJ.
- 76 kJ.
- 100 kJ.
|
The enthapy change is -38 kJ, so the productxs have 38 kJ less energy than the reactants. For the reverse reaction to take place the products must climb by the energy of the forward energy barrier plus the energy by which they are more stable. Therefore reverse activation energy = 38 + 62 = 100 kJ |
Q224-03 The activation energy for the endothermic reaction: NO(g) + O3(g) → NO2(g) + O2(g) is 10.5 kJ. The activation energy for the reverse reaction is:
- Greater than 10.5 kJ.
- The same, 10.5 kJ.
- Less than 10.5 kJ.
- -10.5 kJ.
|
This is an endothermic reaction, so the products have more energy than the reactants. For the reverse reaction to take place the products are already of greater energy than the reactants and this energy value can be subtracted from the activation energy of the forward reaction. Therefore reverse activation energy must be less than 10.5 kJ |
Q224-04 If the activation energy in the forward direction of an elementary step is 52 kJ and the activation energy in the reverse direction is 74 kJ, what is the energy of reaction ΔE for this step?
- 22 kJ
- -22 kJ
- 52 kJ
- -52 kJ
- 126 kJ
|
The difference between the two activation energies gives the enthalpy of the reaction. The sign is dependent on whether or not the products are of lower energy than the reactants. The reverse activation energy is larger than the forward activation energy telling us that the products are of lower energy than the reactants, i.e. it is an exothermic reaction. Therefore, whenever the reverse activation energy is larger than the forward activation energy the reaction is exothermic and the enthalpy of reaction is the difference between the two activation energies, or in this case 52 - 74 = -22 kJ |
Q224-05 Given that a reaction absorbs energy and has an activation energy of 50 kJ/mol, which of the following statements are correct?
- The reverse reaction has an activation energy equal to 50 kJ/mol.
- The reverse reaction has an activation energy less than 50 kJ/mol.
- The reverse reaction has an activation energy greater than 50 kJ/mol.
- The change in internal energy is less than zero.
- The change in internal energy is greater than zero.
- (1) and (4)
- (2) and (4)
- (3) and (4)
- (2) and (5)
- (3) and (5)
|
The reaction absorbs energy = endothermic, i.e. the products are of higher energy than the reactants (statement 5) The forward activation energy = 50 kJ mol-1, so the reverse activation energy cannot be the same. The reverse acivation energy is lower for an endothermic (forward) reaction. Therefore both 2 and 5 are correct |
Q224-06 For the reversible reaction: 2NH3(g) ⇋ N2(g) + 3H2(g); ΔH = -92 kJ and the activation energy equals 335 kJ. The activation energy for the reverse reaction will be:
- -335 kJ
- 92 kJ
- 243 kJ
- 427 kJ
|
For an exothermic reaction the reverse activation energy is greater than the forward activation energy by the magnitude of the enthalpy change. Therefore the reverse activation energy = 335 + 92 = 427 kJ |
Q224-07 If the activation energy of an endothermic reaction is 40 kJ, the activation energy for the reverse reaction is:
- -40 kJ
- 40 kJ
- >40 kJ
- <40 kJ
|
For an endothermic reaction the reverse activation energy is less than the forward activation energy by the magnitude of the enthalpy change. Therefore the reverse activation energy = 40 - enthalpy change = <40 kJ |
Q224-08 A certain reaction has a ΔH = -75 kJ and an activation energy of 40 kJ. A catalyst is found that lowers the activation energy of the forward reaction by 15 kJ. What is the activation energy of the reverse reaction in the presence of this same catalyst?
- 25 kJ
- 60 kJ
- 90 kJ
- 100 kJ
|
The forward reaction must increase in energy by 40 kJ before falling a total of -40 - 75 = -115 kJ to the products. Thus, the backward step from the products to the transition state (highest activated state) = +115 kJ. In the presence of the catalyst the value of 40 kJ is decreased by 15 kJ to 25 kJ, hence the reverse step from products to the highest activation state = 75 + 25 = 100 kJ |
Q224-09 What is the activation energy for the reverse of the reaction below?
N2O4(g) → 2NO2(g) (ΔH = +54.0 kJ and Ea = +57.2 kJ)
- -54.0 kJ
- +3.2 kJ
- +60.2 kJ
- +111.2 kJ
|
For an endothermic reaction the reverse activation energy is less than the forward activation energy by the magnitude of the enthalpy change. Therefore the reverse activation energy = 57.2 - 54 = 3.2 kJ |
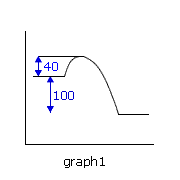
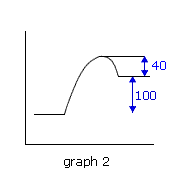
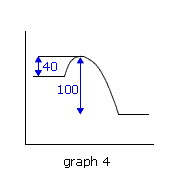
Q224-10 In each of the following potential energy diagrams, the horizontal axis is the reaction coordinate and the vertical axis is potential energy in kJ. Which potential energy diagram best describes a reaction which has an activation energy of 40 kJ and a net energy change (reaction enthalpy) of -100 kJ?
 |
 |
 |
 |
|
The energy change (reaction enthalpy) negative sign indicates that the reaction is exothermic, i.e. graphs 1 and 4, however only graph 1 has an enthalpy change of -100 kJ. It also has the required activation energy of 40 kJ. Therefore graph 1. |