Higher-level only
The rate equation is a general formula that relates the rate of a chemical reaction to the concentration of the reacting species.
Each individual reaction has its own rate equation that must be determined by experiment.
Syllabus ref: R2.2.9Reactivity 2.2.9 - Rate equations depend on the mechanism of the reaction and can only be determined experimentally. (HL)
- Deduce the rate equation for a reaction from experimental data.
Guidance
Tools and links
Relationship between rate and concentration
It may be clearly seen that there is some unspecified relationship between the rate of a chemical reaction and the concentration of the reactants. As a reaction progresses the concentrations of the reactants decreases and so does the rate. Look at the typical graphs below:
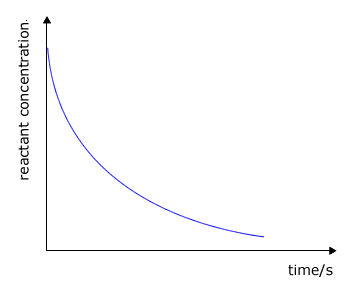 |
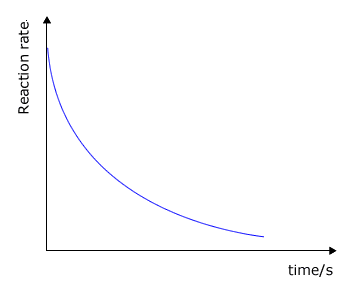 |
|
reactant concentration against
time
|
reaction rate against time
|
The question is, exactly what is the relationship between a specific reactant concentration and the rate? This relationship can only be established by actually performing experiments to obtain data about the rate of the reaction when the concentrations of the reactants are changed (at constant temperature).
The rate expression
Having established that the relationship between rate and concentration can be expressed mathematically, let's consider a reaction in which two hypothetical reactants A and B react together to produce a product 'C'.
A + B → C
The rate of the reaction is dependent on (proportional to) the concentration of A (expressed using square brackets [A]) raised to some unknown power 'x', but it is also dependent on reactant B concentration, [B] raise to a different power 'y'
Therefore:
Reaction rate = constant1 x [A]x and Reaction rate = constant2 x [B]y
Combining these equations this becomes:
Reaction rate = constant (combined from constants 1 and 2) x [A]x x [B]y
This is known as the rate equation:
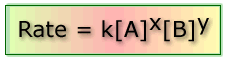
Where: k is the rate constant, x and y are the orders of the reaction with respect to the concentrations of A and B respectively.
Solving the rate equation
The rate equation can only be solved through experiment. For a two component reaction, A + B, the procedure is as follows:
1. A series of experiments are performed, keeping the reactant concentration, A, constant, but changing the concentration of B. The value of [A]x must therefore be constant throughout the experiments and can be combined with the rate constant, k to give the equation:
Rate = constant x [B]y
1. The effect of the change in concentration of B can now be seen on the rate. For example, if the concentration of B is doubled and the rate also doubles, then this means that the value of y = 1; If there is no effect on the rate when [B] is changed, then y = 0; if the rate increases by a factor of 4 when the concentration of B doubles, then the value of y = 1.
3. More experiments are now carried out keeping [B] constant while varying [A]. The value of [B]y is now constant and the rate equation becomes:
Rate = constant x [A]x
4. The effect of the change in concentration of A can now be seen on the rate. For example, if the concentration of A is doubled and the rate also doubles, then this means that the value of x = 1; If there is no effect on the rate when [A] is changed, then x = 0; if the rate increases by a factor of 4 when the concentration of A doubles, then the value of x = 1.
5. Now that values for x and y, the orders of the reaction, have been found, they can be used in any of the experiments to find the value of k, the rate constant, from any of the experimental data.
Rate = k [A]x[B]y
|
Example: For the reaction, X(g) + Y(g) → Z(g) the following kinetic data was obtained:
Calculate the initial rate of the reaction in Exp. 4. Inspection of Experiments 1 and 2 show that the concentration of Y remains constant therefore any effect on the rate is due to change in [X]. BUT as can be seen, when [X] doubles the rate stays the same. This means that change in [X] has no effect on the rate. Therefore: Rate ∝ [X]0 Inspection of Experiments 1 and 3 show that the concentration of X remains constant therefore any effect on the rate is due to change in [Y]. AND as can be seen, when [Y] doubles the rate also doubles. This means that change in [Y] causes the same change in the rate. Therefore: Rate ∝ [Y]1 Combining these two equations gives: Rate = k [X]0[Y]1 As [X] does not affect the rate, we need only look at the change in [Y]. From experiments 3 to 4, [Y] doubles therefore the rate should double as well. Therefore new rate in experiment 4 = 1.48 x 10-2 mol/min |
Worked examples
Q631-01 The following initial rate data were collected for the reaction:aA(g) + bB(g) → cC(g) + dD(g)
|
|
[A]
|
[B]
|
[C]
|
[D]
|
initial rate/ mol dm-3 s-1
|
| Expt 1 |
0.422
|
1.52 x 10-2
|
0
|
0
|
2.72 x 10-5
|
| Expt 2 |
0.638
|
1.21 x 10-2
|
0
|
0
|
4.93 x 10-5
|
| Expt 3 |
0.921
|
1.52 x 10-2
|
0
|
0
|
1.29 x 10-4
|
Find the rate law that best fits this data.
|
In experiments 1 and 3 the concentration of B remains the same, so any effect on the rate is due to concentration of A. Between expts 1 and 3 the concentration of A changes by a factor of 0.921/0.422 = 2.18 At the same time the rate changes by a factor of 4.74 If we square the value 2.18 we get 4.75 therefore the reaction is second order with respect to [A] Considering expt 1 and 2 the concentration of A increases by a factor of 0.638/0.422 = 1.51 We would expect this to have a corresponding effect on the rate of a factor of 1.512 = 2.29 Inspection of the rate change between experiments 1 and 2 reveals an increase by a factor of 4.93/2.72 = 1.81 During this time the concentration of B decreases by a factor of 1.52/1.21
=1.26 The order is therefore first with respect to [B] Therefore the full rate expression is: Rate = k[A]2[B]1 |
Q631-02 For the reaction between hydrogen peroxide and iodide ions in acid solution, represented by the equation:
H2O2 + 3I- + 2H+ → I3- + 2H2O
these kinetic data were gathered:
|
|
[H2O2]
|
[I-]
|
[H+]
|
initial rate/ mol dm-3 s-1
|
| Expt 1 |
0.010
|
0.010
|
0.00050
|
1.15 x 10-6
|
| Expt 2 |
0.020
|
0.010
|
0.00050
|
2.30 x 10-6
|
| Expt 3 |
0.020
|
0.020
|
0.00050
|
4.60 x 10-6
|
| Expt 4 |
0.020
|
0.020
|
0.00100
|
4.60 x 10-6
|
What is the rate law for the reaction?
Answer|
By inspection of expts 1 & 2: ([I-] [H+]
are both constant, therefore any change in the rate is due to [H2O2]) By inspection of expts 2 & 3: ([H2O2] [H+]
= constant, therefore any change in the rate is due to [I-]) By inspection of expts 3 & 4: ([H2O2] [I-]
= constant, therefore any change in the rate is due to [H+]) The rate expression is therefore: Rate = k [H2O2]1[I-]1[H+]0 |
Q631-03 A certain zero-order reaction has a value for the rate constant, k = 0.025 mol dm-3 s-1 for the disappearance of A. What will be the concentration of A after 15 seconds, if the initial concentration is 0.50 mol dm-3?
Answer
|
For a zeroth order reaction, Rate = k[A]0 From this the concentration does not affect the rate. Therefore Rate = k = 0.025 mol dm-3 s-1. After 15 seconds the number of moles used up = 15 x 0.025 = 0.375 mol dm-3 If the initial concentration = 0.5 mol dm-3 Then the mol dm-3 remaining = 0.5 - 0.375 = 0.125 mol dm-3 |
Q631-04 The following initial rate data were collected for the reaction:
aA(g) + bB(g) → cC(g) + dD(g)
| [A] | [B] | initial rate/ mol dm-3 s-1 | |
| Expt 1 | 0.42 | 1.5 x 10-2 | 2.7 x 10-5 |
| Expt 2 | 0.42 | 3.0 x 10-2 | 5.4 x 10-5 |
| Expt 3 | 0.84 | 1.5 x 10-2 | 1.1 x 10-4 |
Find the orders of reaction with respect to [A] and [B], and hence the rate expression.
Answer|
Firstly, remember that the stoichiometry of the equation has NO EFFECT on the rate equation. By inspection of experiments 1 and 2, you can see that the concentration of B doubles while [A] remains constant. Meanwhile, the initial rate doubles. Thus [B] is directly proportional to the initial rate, therefore the order with respect to [B] is one. By inspection of experiments 1 and 3, you can see that the concentration of A doubles while [B] remains constant. However, the initial rate increases by a factor of four. Thus [A] has a squared effect on the initial rate, therefore the order with respect to [A] is 1. The rate equation is therefore: Rate = k [A]2[B]1 |
Q631-05 The following initial rate data were collected for the reaction:
aA(g) + bB(g) → cC(g) + dD(g)
| [A] | [B] | initial rate/ mol dm-3 s-1 | |
| Expt 1 | 0.42 | 1.5 x 10-2 | 2.7 x 10-5 |
| Expt 2 | 0.42 | 3.0 x 10-2 | 5.4 x 10-5 |
| Expt 3 | 0.84 | 1.5 x 10-2 | 2.7 x 10-5 |
Find the orders of reaction with respect to [A] and [B], and hence the rate expression.
Answer|
Firstly, remember that the stoichiometry of the equation has NO EFFECT on the rate equation. By inspection of experiments 1 and 2, you can see that the concentration of B doubles while [A] remains constant. The initial rate meanwhile doubles. Hence, [B] is proportional to the initial rate, therefore the order with respect to [B] is one. By inspection of experiments 1 and 3, you can see that the concentration of A doubles while [B] remains constant. However, the initial rate remains unchanged. Thus [A] has no effect on the initial rate, therefore the order with respect to [A] is 0. The rate equation is therefore: Rate = k [A]0[B]1 |
Q631-06 The following initial rate data were collected for the reaction:
2NO(g) + 2H2(g) → N2(g) + 2H2O(g)
| [NO] | [H2] | initial rate/ mol N2 dm-3 s-1 | |
| Expt 1 | 0.1 | 0.1 | 2.53 x 10-6 |
| Expt 2 | 0.1 | 0.2 | 5.05 x 10-6 |
| Expt 3 | 0.2 | 0.1 | 1.01 x 10-5 |
Find the orders of reaction with respect to [NO] and [H2], and hence the rate expression.
Answer|
Firstly, remember that the stoichiometry of the equation has NO EFFECT on the rate equation. By inspection of experiments 1 and 2, you can see that the [H2] doubles while [NO] remains constant. Meanwhile, the initial rate also doubles. Thus [H2] is directly proportional to the initial rate, therefore the order with respect to [H2] is one. By inspection of experiments 1 and 3, you can see that the concentration of [NO] doubles while [H2] remains constant. Meanwhile, the initial rate increases by a factor of four. Thus [NO] has a squared effect on the initial rate, therefore the order with respect to [NO] is 1. The rate equation is therefore: Rate = k [H2]1[NO]2 |
Q631-07 The following data were obtained for the reaction of nitrogen monoxide gas, NO(g) with oxygen gas to form nitrogen dioxide gas, NO2(g) at 25ºC.
| [NO] | [O2] | initial rate/ mol dm-3 s-1 | |
| Expt 1 | 0.50 | 0.2 | 2.7 x 10-5 |
| Expt 2 | 0.50 | 0.4 | 5.4 x 10-5 |
| Expt 3 | 1.00 | 0.2 | 5.4 x 10-5 |
Find the orders of reaction with respect to [NO] and [O2], and hence the rate expression.
Answer|
Firstly, remember that the stoichiometry of the equation has NO EFFECT on the rate equation. By inspection of experiments 1 and 2, you can see that the [O2] doubles while [NO] remains constant. Meanwhile, the initial rate doubles. Thus [O2] has a proportional effect on the initial rate, therefore the order with respect to [O2] is one. By inspection of experiments 1 and 3, you can see that [NO] doubles while [O2] remains constant. Meanwhile, the initial rate remains also doubles. Thus [NO] has a directly proportional effect on the initial rate, therefore the order with respect to [NO] is 1. The rate equation is therefore: Rate = k [NO]1[O2]1 |
Q631-08 The following data were obtained for the reaction of compounds A and B at constant temperature.
| initial [A]/mol dm-3 | initial [B]/mol dm-3 | initial rate/ mol dm-3 s-1 | |
| Expt 1 | 0.15 | 0.24 | 0.45 x 10-5 |
| Expt 2 | 0.30 | 0.24 | 0.90 x 10-5 |
| Expt 3 | 0.60 | 0.48 | 7.20 x 10-5 |
Find the orders of reaction with respect to [A] and [B], and hence the rate expression.
AnswerFirstly, remember that the stoichiometry of the equation has NO EFFECT on the rate equation. In experiments 1 & 2, the concentration of B is constant. Any effect on the initial rate is due to changing the concentration of A. The concentration of A doubles and at the same time the initial rate doubles. The concentration of A is directly proportional to the rate. The order with respect to [A] = 1. Between experiments 1 & 3 the concentration of A increases by a factor of four. This should engender a similar four-fold increase in the rate. However, in fact, the rate changes from 0.45 x 10-5 to 7.20 x 10-5, representing a 16-fold increase. This can only be due to the change in the concentration of B, which has doubled. Hence, doubling [B] causes an four-fold increase in the rate. The order with respect to [B] = 1. The rate equation is therefore: Rate = k [A]1[B]2 |
Q631-09 The following data were obtained for the reaction of compounds P and Q at constant temperature. The reaction can be represented by the rate expression:
Rate = [P]2[Q]
| initial [P]/mol dm-3 | initial [Q]/mol dm-3 | initial rate/ mol dm-3 s-1 | |
| Expt 1 | 0.20 | 0.30 | 4.80 x 10-3 |
| Expt 2 | 0.10 | 0.10 | |
| Expt 3 | 0.40 | 9.60 x 10-3 | |
| Expt 4 | 0.60 | 19.2 x 10-3 |
Find the concentration of Q in experiment 3.
AnswerThe order with respect to [P] is 1. Hence, the doubling of [P] between experiments 1 and 3 should cause a four-fold increase in the rate. But the rate changes from 4.80 x 10-3 to 9.60 x 10-3, which represents only a two-fold increase. The effect of [Q] on the rate is one of direct proportionality. Hence [Q] must have halved from Expt 1 to 3 to reduce the rate by a factor of 1. Hence, the value of [Q] in experiment 3 = 0.15 mol dm-3 |
Q631-10 The bromination of acetone that occurs in acid solution is represented by this equation.
CH3COCH3(aq) + Br2(aq) → CH3COCH2Br(aq) + H+(aq) + Br-(aq)
These kinetic data were obtained for given reaction concentrations.
|
Initial Concentrations,
/ mol dm-3 |
Initial Rate of disappearance of Br2,
/ mol dm-3 s-1 |
||
| [CH3COCH3] |
[Br2]
|
[H+]
|
|
|
0.30
|
0.050
|
0.050
|
5.7 x 10-5
|
|
0.30
|
0.10
|
0.050
|
5.7 x 10-5
|
|
0.30
|
0.10
|
0.10
|
1.1 x 10-4
|
|
0.40
|
0.050
|
0.20
|
3.1 x 10-4
|
Based on these data, what is the rate equation?
Answer|
In experiments 1 & 2 [CH3COCH3] and [H+]
are constant: In experiments 2 & 3 [CH3COCH3] and [Br2]
are constant: In experiments 3 & 4 nothing is constant but we already know that
the order is 0 wrt [Br2]: The concentration of [CH3COCH3] increases by a factor of 4/3. Multiplying this factor by the value expected of the rate, 2.4 x 10-4, gives 3.2 which is the rate in experiment 4. The order wrt [CH3COCH3] is 1. Therefore the rate expression is: Rate = k [CH3COCH3]1[Br2]0[H+]1 |
| Now test yourself |