Standard level
The equilibrium law describes how the position of equilibrium depends on the concentrations of the reactants and the products of the equilibrium reaction.
Syllabus ref: R2.3.2Reactivity 2.3.2 - The equilibrium law describes how the equilibrium constant, K, can be determined from the stoichiometry of a reaction.
- Deduce the equilibrium constant expression from an equation for a homogeneous reaction.
Guidance
Tools and links
The equilibrium expression
The concentrations of reactants and products are constant at equilibrium. This means that their ratio can be expressed as a simple mathematical formula:
For any reaction of the form:
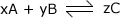
The equilibrium expression is given by:
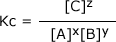
Where:
- [A] = the concentration of component A,
- [B] = the concentration of component B, etc.
Kc is called the equilibrium constant with respect to the concentration of the reactants and products (that's why there is a subscript 'c' following the letter traditionally used for a constant value, 'K'.
NOTE In the above example, the coefficients of the equation appear in the equilibrium expression as powers to which the concentrations of the reactants and products are raised.
Example:Show the equilibrium expression, with respect to concentrations, for the following reversible reaction:
H2(g) + I2(g) ⇋ 2HI(g)
Kc is given by the product concentrations raised to the power of their coefficients, divided by the reactants concentrations raised to the power of their coefficients:
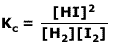
Solids and liquids
Only those substances that appear in the equilibrium in a form that can be expressed in terms of concentration can appear in the equilibrium expression. This means to say that solids NEVER appear and liquids only appear if they are part of a homogeneous system.
Example
Show the expression for the equilibrium that exists between iron, water, hydrogen and iron(III) oxide at elevated temperatures:
2Fe(s) + 3H2O(g) ⇋ Fe2O3(s) + 3H2(g)
Inspection of the equation reveals that both iron and iron oxide are in the solid states, i.e. they do not have a concentration. This means that they cannot appear in the equilibrium expression, therefore:

Worked examples
|
Kc =
|
[O2]5[NH3]4
|
|
[NO]4[H2O]6
|
Which equation corresponds to this equilibrium expression?
- 4NH3 + 5O2 ⇋ 4NO + 6H2O
- 4NO + 6H2O ⇋ 4NH3 + 5O2
- 8NH3 + 10O2 ⇋ 8NO + 12H2O
- 2NO + 3H2O ⇋ 2NH3 + 5/2O2
|
The equilibrium law: the equilibrium constant is equal to the concentration of the products raised to the power of their coefficients, divided by the concentration of the reactants raised to the power of their coefficients. Equation B: 4NO + 6H2O ⇋ 4NH3 + 5O2 is the correct response |
Q723-02 Which of the following is the equilibrium expression for the reaction below?
I2(g) + 3Cl2(g) ⇋ 2ICl3(g)
|
A.
|
|
|||
|
B.
|
|
|||
|
C.
|
|
|||
|
D.
|
|
Answer
|
The products concentrations raised to the power of their reaction coefficients, divided by the concentration of the reactants raised to the power of their reaction coefficients. Correct response - D |
Q723-03 The reaction between methane and hydrogen sulfide is represented by the equation below:
CH4(g) + 2H2S(g) ⇋ CS2(g) + 4H2(g)
What is the equilibrium expression for the reaction
- [CS2][H2] / [CH4][H2S]
- 4[CS2][H2] / 2[CH4][H2S]
- [CS2]+4[H2] / [CH4]+2[H2S]
- [CS2][H2]4 / [CH4][H2S]2
|
The products concentrations raised to the power of their reaction coefficients, divided by the concentration of the reactants raised to the power of their reaction coefficients. Correct response - D |
Q723-04 What is the equilibrium expression for the following reaction:
N2(g) + 3H2(g) ⇋ 2NH3(g)
| A. |
|
||||
| B. |
|
||||
| C. |
|
||||
| D. |
|
Answer
|
The products concentrations raised to the power of their reaction coefficients, divided by the concentration of the reactants raised to the power of their reaction coefficients. Correct response - D |
Q723-05 In the reaction of iron(III) oxide with hydrogen at 500ºC the following equilibrium is established:
Fe2O3(s) + 3H2(g) ⇋ 2Fe(s) + 3H2O(g)
Write the equilibrium expression for this reaction.
Answer|
As solids do not appear in the equilibrium expression:
|
.gif)