Higher-level only
The mode of action of indicators is discussed in this section.
Syllabus ref: R3.1.14Reactivity 3.1.14 - Acid–base indicators are weak acids, where the components of the conjugate acid–base pair have different colours. (HL)
- The pH of the end point of an indicator, where it changes colour, approximately corresponds to its pKa value.
- Construct equilibria expressions to show why the colour of an indicator changes with pH.
Guidance
- The generalized formula HInd(aq) can be used to represent the undissociated form of an indicator.
- Examples of indicators with their pH range are given in the data booklet.
- Include universal indicator as a mixture of many indicators with a wide pH range of colour change.
Tools and links
- Tool 1, Inquiry 2, Reactivity 3.2 - What are some of the similarities and differences between indicators used in acid–base titrations and in redox titrations?
Indicator action
An indicator is a weak acid (or base) which has a different colour in the ionic and molecular forms.
When a weak acid is in solution its molecular form predominates and this colour is seen. However, as the equilibrium involves hydrogen ions, then it is pH sensitive. Addition of hydrogen ions forces the equilibrium in the direction of the molecular form (left hand side) whereas when base is added to the equilibrium it moves in the direction of the ionic form (the conjugate base) and this colour is seen.
CH3COOH ⇋ CH3COO- + H+
Hence, in this equilibrium, addition of hydrogen ions will push the equilibrium in the direction of the CH3COOH molecules, whereas addiiton of base will react with the hydrogen ions from the equilibrium and pull the equilibrium to the right hand side. In the case of ethanoic acid there will be no colour change so it could not be used as an indicator.
We generally represent the weak acids that are indicators by the formula HIn, meaning that this is the molecular form of the acid that is going to lose the hydrogen ion. In this case the ionic form will be In-. For the indicator to be useful the colour of HIn must be different from In-.
If we use phenolphthalein as an example, then the molecular form of phenolphthalein (HIn) has no colour. Hence in acidic solution the equilibrium is pushed to the left hand side and the phenolphthalein is in the colourless molecular form.
HIn ⇋
H+ + In-

low pH (high concentration of H+ ions) pushes equilibrium to the left
If the phenolphthalein is in basic solution then the excess OH- ions react with the H+ ions from the equilibrium pulling it to the right hand side and the colour of the In- ion is seen, which is red in the case of phenolphthalein.
HIn ⇋ H+ + In-

high pH (high concentration of OH- ions) pulls equilibrium to the right
pKa of indicators
As indicators have a different coulour when in the molecular form to when in the ionic form it seems logical to assume that the point of changing colour will be when there is 50% of both forms present i.e. the equilibrium HIn ⇋ H+ + In- lies exactly in the middle.
If we apply the equilibrium law to this situation then:
|
And when the equilibrium lies exactly in the centre then:
| [In-] = [HIn] |
In which case we can cancel them out from the equation to give:
Ka = H+ |
And therefore:
| pKa = pH |
The consequence of this answer is that the indicator will change colour when the pH is the same value as its pKa value. Hence, indicators have different regions of operation. As the change in pH is usually large at the equivalence point this means that provided the pH change takes place through the pKa of the indicator then it can be used for a titration.
Example:
The pKa of phenolphthalein is 9.3 and may be usefully employed over the pH range 8.2 - 10.0.
In the titration of a strong acid with a strong base there is a change from pH3 to pH 11 with the addition of 1 drop of reagent and consequently the colour of the phenolphalein will change as well.
However, with the titration of a strong acid and a weak base the equivalence point will not correspond to this region of pH change and the pH does not pass through 8 - 10.
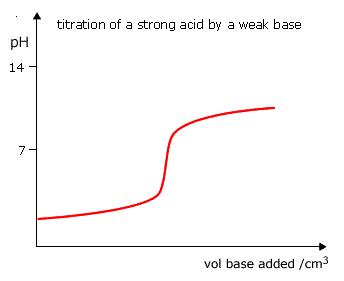
Phenolphthalein cannot be used for this type of titration.
Choice of indicator
The indicator must be chosen to suit the titration being performed.
Titrations involving strong acids and bases can be performed using any indicator as the pH change at the equivalence point is large (from 3 to 11 or vice versa) - most indicators operate within this range.
Titrations involving strong acids and weak bases have an equivalence point in the acidic region of the pH scale. The choice of indicator will depend on the actual expected pH at the equivalence point. It makes sense to select an indicator with a pKa right in the middle of the pH change at the equivalence point. In practice, there are few indicators in common use. For strong acids and weak bases methyl orange is the indicator of choice.
| Indicator |
pKa
|
Useful range
|
|---|---|---|
| Methyl orange |
3.7
|
3.1 - 4.4
|
| Bromophenol blue |
4.0
|
3.0 - 4.6
|
| Methyl red |
5.1
|
4.2 - 6.3
|
| Bromothymol blue |
7.0
|
6.0 - 7.6
|
| Phenol red |
7.9
|
6.8 - 8.4
|
| Phenolphthlein |
9.3
|
8.2 - 10.0
|
Summary
- Titrations involving weak bases and strong acids use methyl orange
- For weaK acids and strong bases use phenolphthalein
- Strong acids and strong bases can use any indicator
- Weak acids and weak bases cannot be titrated.