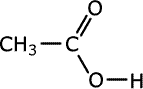
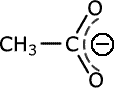
Standard level
Several organic functional groups can undergo reduction. These include aldehydes (alkanals), ketones (alkanones) and carboxylic acids. Aldehydes and ketones are both carbonyl compounds, that is they contain alkyl chains attached to a C=O group. The difference between them is that the aldehyde also has a hydrogen attached to the carbonyl group. This confers aldehydes with sightly different properties to ketones.
Syllabus ref: R3.2.10Reactivity 3.2.10 - Functional groups in organic compounds may undergo reduction.
- Deduce equations to show reduction of carboxylic acids to primary alcohols via the aldehyde, and reduction of ketones to secondary alcohols.
Guidance
- Include the role of hydride ions in the reduction reaction.
- Names and formulas of specific reducing agents, and the mechanisms of reduction, will not be assessed.
Tools and links
- Structure 3.1 - How can oxidation states be used to show that the following molecules are given in increasing order of oxidation: CH4, CH3OH, HCHO, HCOOH, CO2?
Aldehydes
Aldehydes have the general formula CxH2x+1CHO, although the first member of the homologous series is methanal, HCHO (x=0).
The carbonyl group has a pair of electrons in a pi orbital between the carbon atom and the oxygen of the carbonyl group. This is a polarised system due to the high electronegativity of the oxygen atom.

Oxidation
This reactivity of the carbonyl group means that aldehydes can be oxidised easily to carboxylic acids. This is carried out using potassium dichromate(VI) in acidic solution under reflux (to prevent loss of the volatile aldehyde).
Reduction
In section section R3.2.9 the oxidation of alcohols to aldehydes was described:

This reaction can be made to go in the reverse direction using a strong reducing agent.
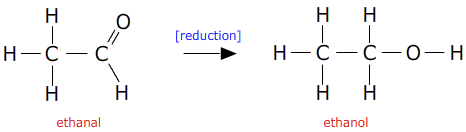
Suitable reducing agents are lithium aluminium hydride (lithium tetrahydroaluminate) or sodium borohydride (sodium tetrahydroborate).
Lithium aluminium hydride (lithium tetrahydroaluminate)
Lithium aluminium hydride, LiAlH4, is a highly reactive reducing agent that must be used in non-aqueous solutions, for example, ethoxyethane (ether). This is because it reacts vigorously with water.
The reaction is carried out in two stages:
- 1 heating with LiAlH4 in ether forming a complex
- 2 decomposition of the complex formed in 1 by adding aqueous acid to give the required product
Sodium borohydride (sodium tetrahydroborate)
Sodium borohydride, NaBH4, has an advantage over lithium aluminium hydride in that it is not decomposed by water at high pH and hence can be used in aqueous solutions.
It is, however, a less powerful reducing agent, although perfectly adequate for reduction of aldehydes and ketones.
Ketones
Ketones have the general formula CxH(2x+2)CO, arranged with the two alkyl groups either side of the carbonyl group.
Ketones cannot undergo oxidation without cleavage of a carbon - carbon bond, which would require extreme conditions. With potassium dichromate(VI) in acidic solution there is no reaction.
Reduction of ketones
In section R3.2.9 the oxidation of secondary alcohols was shown to produce ketones. Using a suitable reducing agent the reverse reaction is also possible. Hence, ketones are reduced to secondary alcohols by strong reducing agents:

As with aldehydes, both lithium aluminium hydride and sodium borohydride (sodium tetrahydroborate) may be used.
Carboxylic acids
Carboxylic acids can be reduced to aldehydes, and further to primary alcohols, by strong reducing agent such as lithium aluminium hydride (lithium tetrahydroaluminate). This reagent is used in ethoxyethane (diethyl ether) as it is very water sensitive. The complex formed is then hydrolysed by dilute acid to the final product.
CH3COOH + [LiAlH4] → CH3CHO
CH3CHO + [LiAlH4] → CH3CH2OH
Worked examples
R3210-01 What is produced when ethanoic acid reacts with magnesium ribbon?
Answer|
This is a typical acid + active metal reaction:
|
R3210-02 When methanoic acid is tested for with sodium hydrogen carbonate, which ionic product is formed?
Answer|
Methanoic acid reacts with sodium hydrogen carbonate to make sodium methanoate + carbon dioxide + water. |
R3210-03 Arrange the following acids in order of increasing acidity.
- methanoic acid
- propanoic acid
- chloroethanoic acid
|
Chloroethanoic acid has an electron withdrawing chlorine, making it the strongest. Methanoic acid, HCOOH, has no electron withdrawing or inducing groups, whereas the propanoic acid has an electron inducing alkyl group attached to the COOH. The order of increasing acidity is propanoic acid < methanoic acid < chloroethanoic acid |
R3210-04 Benzoic acid is insoluble in cold water due to the bulky hydrophobic benzene group. However, when benzoic acid is stirred with sodium hydroxide solution it dissolves easily. Explain this observation.
Answer|
Benzoic acid reacts with sodium hydroxide making an ionic salt, sodium benzoate that is readily soluble. |
R3210-05 Why is concentrated sulfuric acid added to the reaction mixture when ethanoic acid reacts with methanol?
Answer|
The reaction is an equilibrium and the concentrated sulfuric acid acts as both a catalyst for the reaction and a dehydrating agent to remove the water formed, pushing the equilibrium in the forward direction. |
R3210-06 What is the name of the product formed when methanoic acid reacts with propanol?
Answer|
This is an esterification reaction, the product is propyl methanoate. |
R3210-07 In an experiment to determine the relative molecular mass of an acid by measing the volume of its vapour, a student found that ethanoic acid had a relative molecular mass of 120. How can this be explained?
Answer|
Ethanoic acid has the formula CH3COOH. The relative mass of this molecule is 60. The apparent relative mass is caused by the ethanoic acid dimerising (two molecules per dimer) even in the vapour state. The value of 120 can be explained by dimerisation. |
R3210-08 Which compound is formed by reduction of ethanoic acid using excess lithium aluminium hydride?
Answer|
Reduction of ethanol first produces ethanal and then ethanol |
R3210-09 Which carboxylic acid is produced by oxidation of butan-1-ol using excess potassium dichromate(VI) in dilute sulfuric acid?
Answer|
Oxidation of butan-1-ol produces butanal and then further oxidation produces butanoic acid. |
R3210-10 Spectroscopic studies show that the carbon-oxygen bond lengths in ethanoic acid are different, whereas the carbon oxygen bond lengths in the ethanate ion are the same. How is this explained?
Answer|
In carboxylic acids the carbon-oxygen bond lengths are different because one of the bonds is a carbon-oxygen single bond and the other is a carbon-oxygen double bond. Single bonds are longer than double bonds. In the carboxylate ion, there is resonance between the two bonds, with the overall effect of delocalisation of the electron pair over the whole COO- system. The two carbon-oxygen bonds are therefore identical and equal length.
|