Standard level
Certain organic functional groups are able to undergo redox reactions. Alcohols and aldehydes can be oxidised, while carboxylic acids, aldehydes and ketones can be reduced. In this section we examine the redox reactions of certain organic compounds.
Syllabus ref: R3.2.9Reactivity 3.2.9 - Functional groups in organic compounds may undergo oxidation.
- Deduce equations to show changes in the functional groups during oxidation of primary and secondary alcohols, including the two-step reaction in the oxidation of primary alcohols.
Guidance
- Include explanation of the experimental set-up for distillation and reflux.
- Include the fact that tertiary alcohols are not oxidized under similar conditions.
- Names and formulas of specific oxidizing agents, and the mechanisms of oxidation, will not be assessed.
Tools and links
- Structure 3.2 - How does the nature of the functional group in a molecule affect its physical properties, such as boiling point?
- Reactivity 1.3 - What is the difference between combustion and oxidation of an alcohol?
- AHL Structure 3.1 - Why is there a colour change when an alcohol is oxidized by a transition element compound?
Alcohols (alkanols)
Alcohols are an homologous series of organic compounds containing the hydroxyl group. The commonly known 'alcohol' is actually more correctly known as ethanol.
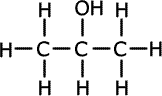
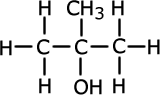
There are three types of alcohol characterised by slight differences in the structural position of the hydroxyl functional group. These are known as primary, secondary and tertiary alcohols.
 |
 |
 |
|
Propan-1-ol (primary)
|
Propan-2-ol (secondary)
|
Methylpropan-2-ol (tertiary)
|
Combustion
Combustion can be considered an oxidation reaction as the oxidation state of the carbon atoms in the alcohol increases to +4 in complete combustion.
Alcohols, like the majority of organic compounds, burn in air or oxygen. The products of complete combustion are carbon dioxide and water. In a restricted supply of air the products will include carbon monoxide and possibly carbon as well as carbon dioxide and water.
Complete combustion:
methane + oxygen → carbon dioxide + water
CH4 + 2O2 → CO2 + 2H2O
Oxidation
Alcohols are oxidised by strong oxidising agents such as potassium dichromate (VI) in acidic solution. The products depend upon the nature of the alcohol.
- Primary alcohols produce aldehydes
- Secondary alcohols produce ketones
- Tertiary alcohols are not easily oxidised (no reaction with potassium dichromate in acidic solution)
This reaction may be used to differentiate between 1º, 2º and 3º alcohols.
Oxidation of primary alcohols first produces aldehydes, which may be further oxidised to carboxylic acids under the same conditions. This means that to obtain the aldehyde from this reaction it must be removed as soon as it is formed. This is done by making use of the fact that aldehydes are more volatile than the corresponding alcohol and carboxylic acid.
Oxidation of secondary alcohols produces ketones (alkanones). Ketones can't be further oxidised, so this reaction is carried out in reflux apparatus.
Summary - The oxidation of alcohols
Note: A knowledge of specific oxidising agents is no longer required for first examinations in 2025
Worked examples
QR329-01 What is the organic product of the reaction between ethanol and ethanoic acid- CH3CHO
- CH3COOCH3
- CH3CH2COOCH3
- CH3COOCH2CH3
|
This is an esterification reaction. The product is CH3COOCH2CH3 |
QR329-02 What is the final product formed when CH3CH2OH is refluxed with acidified potassium dichromate(VI) ?
- CH3CHO
- CH2=CH2
- CH3COOH
- HCOOCH3
|
This is an oxidation reaction. The final product of oxidation of a primary alcohol is a carboxylic acid, CH3COOH |
QR329-03 The compound CH3CH2OH is distilled with excess acidified potassium dichromate(VI) solution What is the name of the functional group of the final organic product formed?
- Alkanal
- Alkanone
- Alkanoic acid
- Alkanol
|
This is an oxidation reaction. Distillation removes the aldehyde as soon as it is formed, CH3CHO and prevents further oxidation. |
QR329-05 Which compound is converted to butanal by acidified potassium dichromate(VI) solution?
- Butan-1-ol
- Butan-2-ol
- Butanone
- Butanoic acid
|
Primary alcohols are oxidised to aldehydes. The compound that is converted to butanal is butan-1-ol |
QR329-06 What is the major product formed when a mixture of CH3CH2OH and concentrated H2SO4 is heated strongly?
- CH3CH3
- CH3CH2SO4
- CH3COOH
- CH2CH2
|
Concentrated sulfuric acid is a dehydrating agent. Dehydration of alcohols produces alkenes. The final product is CH2CH2 |
QR329-08 Which substance in not readily oxidised by acidified potassium dichromate(VI) solution?
- Propan-1-ol
- Propan-2-ol
- Propanal
- Propanone
|
Tertiary alcohols are not readily oxidised, neither are ketones, propanone. |
QR329-09 Consider the following compounds:
- I - CH3CH2CH(OH)CH3
- II - CH3CH(CH3)CH2OH
- III - (CH3)3COH
The compounds are treated separately with acidified potassium dichromate(VI). Which will produce a colour change from orange to green?
- I and II only
- I and III only
- II and III only
- I, II and III
|
The colour change from orange to green is an indication that oxidation has taken place, therefore the compounds that could be oxidised are I and II only. Compound III is a tertiary alcohol and cannot be oxidised. |