Standard level
Free-radicals are neutral species that contain unpaired electrons.
Syllabus ref: R3.3.1Reactivity 3.3.1 - A radical is a molecular entity that has an unpaired electron. Radicals are highly reactive.
- Identify and represent radicals, e.g. ●CH3 and Cl●.
Guidance
Tools and links
- Structure 2.1 - How is it possible for a radical to be an atom, a molecule, a cation or an anion? Consider examples of each type.
Electronic configuration of atoms and molecules
One of the markers of thermodynamic stability in atoms and molecules is the presence of complete outer shells. In atoms, this is sometimes known as the octet rule.
The atoms of the elements of group 18 (the Noble or Inert gases) all have complete outer shells. These elements are very unreactive, only the heavier members can form compounds and only then with fluorine and/or oxygen.
In the section on Lewis structures, we met xenon tetrafluoride, XeF4, as an example of a compound whose molecules have an expanded octet.
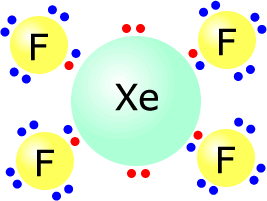
In general, atoms bond together forming covalent compounds in which all atoms have complete octets. This does not apply to hydrogen, whose "1s" orbital can only accomodate a maximum of 2 electrons.
In ionic bonding, all ions have full outer shells.
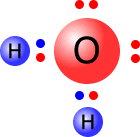
Single electron species
Atoms
Many atoms contain unpaired electrons and as a result, are highly reactive. These are also called free-radicals. Atoms solve this reactivity issue by combining together pairing up their unpaired electrons forming molecules.
Hence hydrogen, with one unpaired electron, exists as hydrogen molecules, H2, in which the electron is paired.
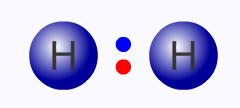
If enough energy is applied to a covalent bond, it can break evenly, with one electron remaining with each atom. This kind of bond fission is said to be "homolytic" and the species formed are called free-radicals.
To do this to hydrogen molecules requires a lot of energy, but for some molecules such as chlorine, Cl2, the amount of energy needed falls into the high energy region of the electromagnetic spectrum. The Cl-Cl bond energy is 242 kJ mol-1
Homolytic fission of chlorine
Cl2 ⇋ 2Cl•
The chlorine free-radicals formed are represented by the symbol for chlorine and a dot, indicating the single unpaired electron.
The chlorine atom
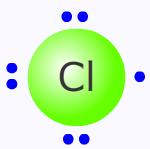
It should be emphasised at this point that a chlorine atom and a chlorine free-radical are one and the same thing. They are identical.
Molecules
Molecules can also exist with single electrons. This is the case for some common substances.
Dinitrogen tetroxide, N2O4, contains a nitrogen-nitrogen single bond that can undergo homolytic fission with relatively low energy input. This causes the dinitrogen tetroxide to dissociate (break apart) into two electron deficient molecules of nitrogen(IV) oxide, •NO2.
The N-N bond energy is 57 kJ mol-1.
The nitrogen(IV) oxide molecule
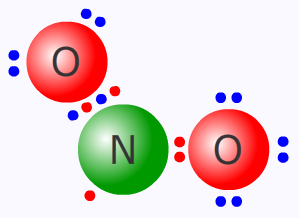
Dissociation of dinitrogen tetroxide
N2O4 ⇋ 2•NO2
Another well-known free-radical compound is chlorine monoxide, ClO•, implicated in the breakdown of the ozone layer by chlorine free-radicals from chlorofluorocarbons in the atmosphere.