Standard level
A nucleophile, literally "seeking positive charge" is a reactant that forms a bond to its reaction partner (the electrophile) by donating both bonding electrons.
Syllabus ref: R3.4.1Reactivity 3.4.1 - A nucleophile is a reactant that forms a bond to its reaction partner (the electrophile) by donating both bonding electrons.
- Recognize nucleophiles in chemical reactions.
Guidance
- Both neutral and negatively charged species should be included.
Tools and links
Nucleophiles
A nucleophile is a chemical species that donates an electron pair to form a chemical bond in a reaction. Nucleophiles are typically rich in electrons and have lone pairs or π bonds that can be donated to an electrophile.
Common nucleophiles include negatively charged ions like hydroxide (OH⁻), cyanide (CN⁻), and halides (Cl⁻, Br⁻, I⁻), as well as neutral molecules with lone pairs such as ammonia (NH3) and water (H2O).
Lewis structure of ammonia
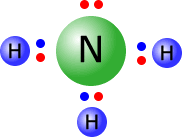
In organic chemistry, nucleophiles play a crucial role in substitution and addition reactions, where they attack positively charged or electron-deficient atoms or molecules to form new bonds. The strength of a nucleophile, known as its nucleophilicity, depends on various factors, including charge, electronegativity, solvent, and the presence of electron-donating groups.
Generically, nucleophiles can be represented by the symbol :Nu.
Nucleophilic processes
Nucleophilic reactions may be substitution or addition, although the IB course does not require the latter.
Nucleophilic substitution involves attack by the lone pair on the nucleophile to an electron deficient atom of another species, followed by loss of another particle. In general:
Electrophiles (that attract nucleophiles electron pairs) work well with nucleophiles if they also have a good "leaving group".
Nu: + RX → Nu-R + X
In the equation above the species "X" is the leaving group.